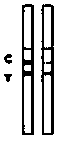Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
54 results about "Immunologic Procedures" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
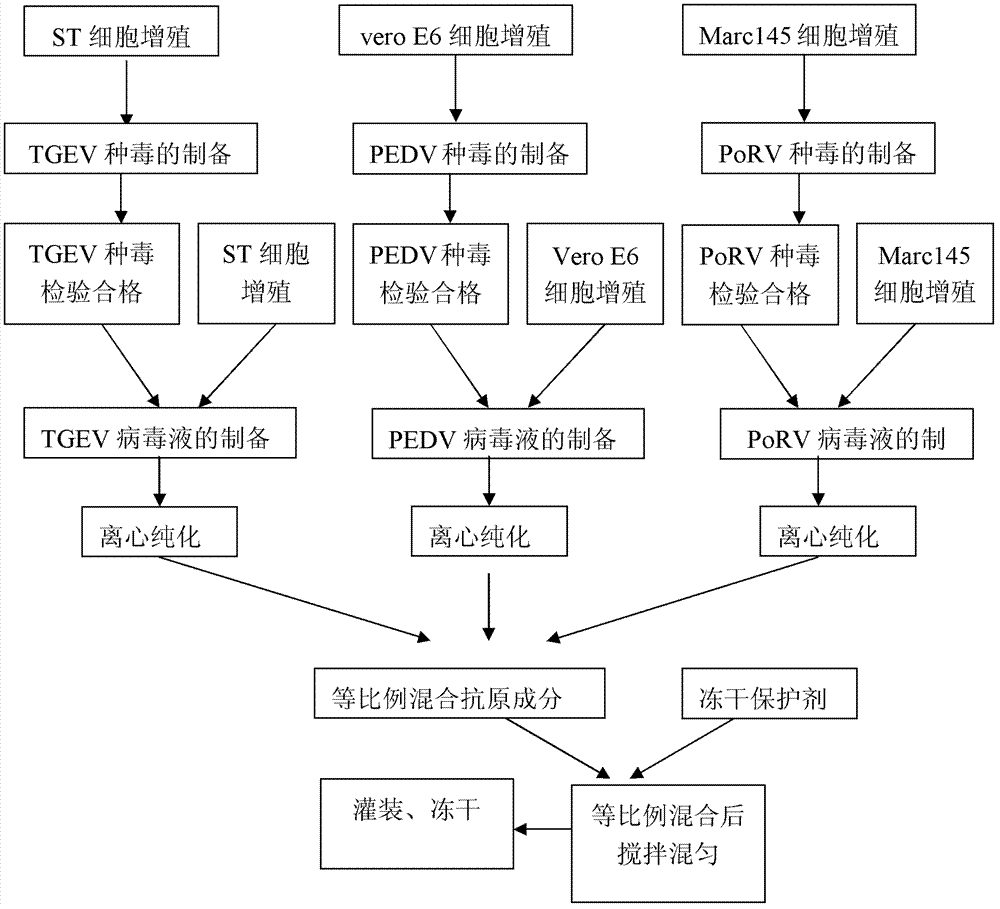
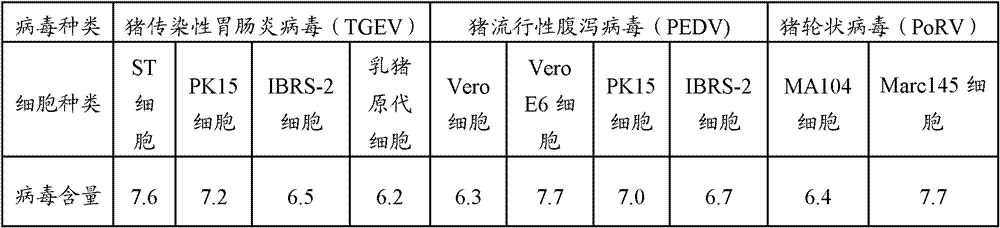
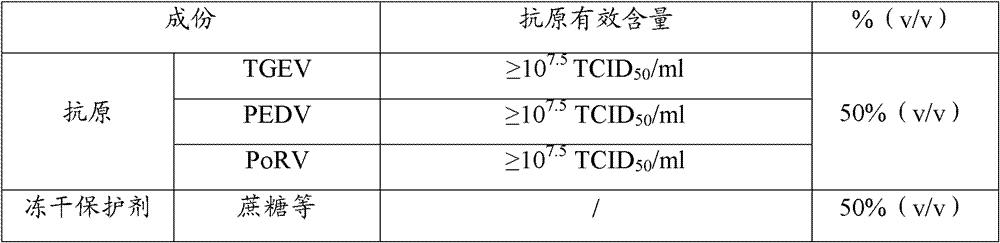
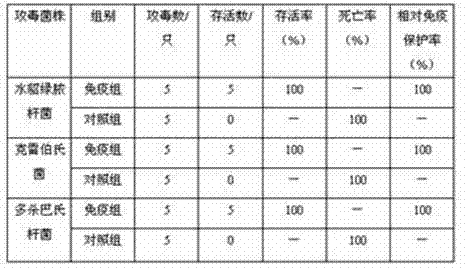
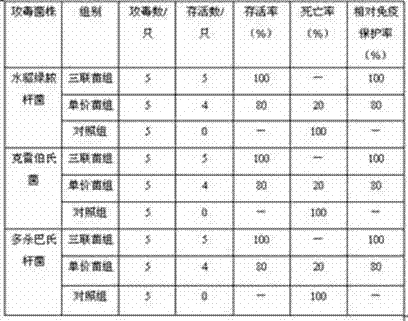
Triple live vaccine for swine transmissible gastroenteritis virus, swine epidemic diarrhea virus and swine rotavirus
ActiveCN102949718AReduce immune efficiencyReduced immune potencyViral antigen ingredientsAntiviralsEpidemic diarrheaRotavirus RNA
The invention provides a triple live vaccine for a swine transmissible gastroenteritis virus, a swine epidemic diarrhea virus and a swine rotavirus and a preparation method thereof. The content of the three viruses is not less than 107.5 TCID50 (Tissue Culture Infectious Dose 50) / mL, and the volume ratio is 1:1:1. The triple live vaccine provided by the invention solves the problem that a multiple vaccine for effectively preventing and treating such three diseases as swine transmissible gastroenteritis, swine epidemic diarrhea and the swine rotavirus is not available on the current market, and especially realizes the prevention and control on the swine rotavirus. Compared with the existing method of inoculating with three simplex vaccines to prevent such three transmissible diseases, the triple live vaccine provided by the invention is economical to use, simplifies the immunization procedure and lowers the epidemic prevention cost, thereby providing a new simple and convenient immunization way for farms in China.
Owner:PU LIKE BIO ENG
Polyclonal antibody of phosphorylating and corresponding non-phosphorylating protein in application for preparing reagent for disease diagnosis, and preparation method
A method for preparing multicloning antibody includes synthesizing polypeptide, connecting the coupling agent with keyhole limpet hemocyanin for forming hapten ¿C carrier protein system, immunizing rabbit then picking up its blood for separating out serum, purifying out multicloning antibody of anti ¿C specific site amino acid phosphorylation protein and anti ¿C corresponding non phosphorylation protein after two steps of antigen affinity chromatography .
Owner:南京川博生物技术有限公司
Immunogenic compositions for protection against Chlamydial infection
InactiveUS20050065106A1Improve efficacySugar derivativesChlamydiaceae ingredientsNucleotideProtection sex
Owner:MURDIN ANDREW +1
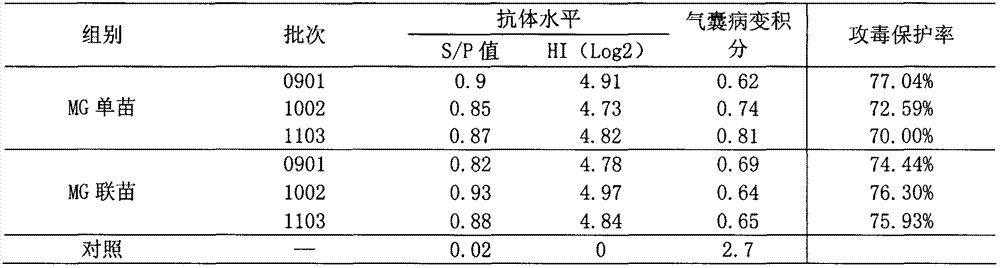
Efficacy test method of mycoplasma gallisepticum inactivated vaccine and application thereof
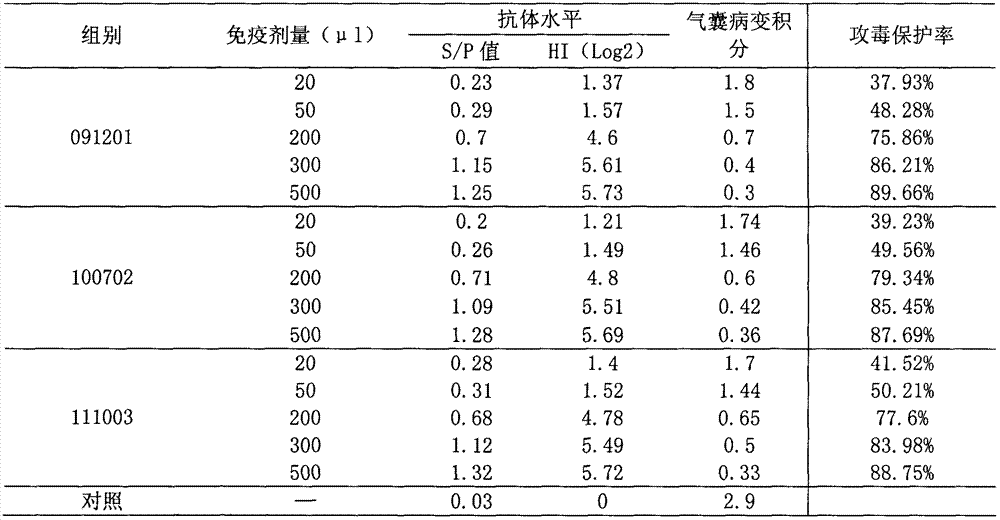
The invention relates to the technical field of animal biological products and discloses an efficacy test method of mycoplasma gallisepticum inactivated vaccine. Simultaneously, the efficacy test method disclosed by the invention can also be applied to quality control of the vaccine, in particular comprising the determination of immune toxicity attack protection rate and vaccine quality standard, measurement of immunization deadline and clinical monitoring. Experiments prove that the animal toxicity attack test is replaced by a serology detection method (comprising ELISA (Enzyme-Linked Immuno Sorbent Assay) and HI (Hemagglutination Inbition)); the efficacy test method is simple, convenient, rapid, accurate in result and good in repeatability and specificity; due to the establishment of the efficacy test method, subjectivity for checking airbag pathological change integrals after attacking toxicity is reduced; toxicity dispersion is avoided; a basis for establishing a rational immune program is also provided; and the efficacy test method has general popularization significance.
Owner:兆丰华生物科技(南京)有限公司
Triple inactivated vaccine for porcine epidemic diarrhea, swine transmissible gastroenteritis and porcine delta coronavirus and preparation method of triple inactivated vaccine
ActiveCN107899007AImmunization method is simpleAddressing the absence of vaccinesSsRNA viruses positive-senseViral antigen ingredientsDiseaseEpidemic diarrhea
The invention provides a triple inactivated vaccine for porcine epidemic diarrhea (PED), porcine transmissible gastroenteritis (TGE) and porcine delta coronavirus (PDCoV) and a preparation method of the triple inactivated vaccine, before inactivation, the contents of the three viruses are greater than or equal to 10<7.0>TCID50 / mL, and after inactivation, the volume ratio of the antigens is 1 to 1to 1. With the triple inactivated vaccine disclosed by the invention, the problem that an effective multivalent vaccine for preventing and treating three diseases including porcine epidemic diarrhea (PED), porcine transmissible gastroenteritis (TGE) and porcine delta coronavirus (PDCoV) is not available on the market is solved, especially, the problem that the vaccine for the porcine delta coronavirus (PDCoV) epidemic in recent years is not available is solved. The triple inactivated vaccine provided by the invention is economical and practical, the immunizing procedure is simplified, the epidemic prevention cost can be effectively reduced, and a novel method for simultaneously preventing the occurrence of the three diseases is provided for domestic breeding enterprises.
Owner:YULIN UNIV +1
Swine fever and porcine pseudorabies bivalent vaccine as well as preparation method and application thereof
InactiveCN103505724ASolve the problem of low early potencyImprove securityAntiviralsAntibody medical ingredientsDiseaseRabies
The invention provides a swine fever and porcine pseudorabies bivalent vaccine. The swine fever and porcine pseudorabies bivalent vaccine contains at least one swine fever virus antigen and at least one porcine pseudorabies virus antigen, wherein the two antigens coordinate well, are excellent in immune effect and can promote each other. The swine fever and porcine pseudorabies bivalent vaccine is simple in preparation method, is convenient and efficient in immunization and has the advantages that immunization cost is reduced, an immunization procedure is simplified and economy and reliability are realized compared with a vaccine which can be used for preventing and treating more than two diseases only when immunization is carried out in steps and at least two injections are taken and an immune method of the vaccine in the prior art. The immune effect of the swine fever and porcine pseudorabies bivalent vaccine is better than that of a single vaccine and better in safety and avoids adverse effects caused by multiple immunizations. Besides, the invention also provides a simple testing method for determining swine fever effect in the bivalent vaccine by adopting an indirect immunofluorescence method, so that quality of bivalent live vaccines in each batch is guaranteed, and economic benefit is obviously increased.
Owner:PU LIKE BIO ENG
Trivalent inactivated vaccine of porcine reproductive and respiratory syndrome virus, porcine circovirus type 2, and porcine pseudorabies virus and preparation method thereof
InactiveCN102973932AIncrease productionImprove securityViral antigen ingredientsAntiviralsImmune effectsMultivalent Vaccine
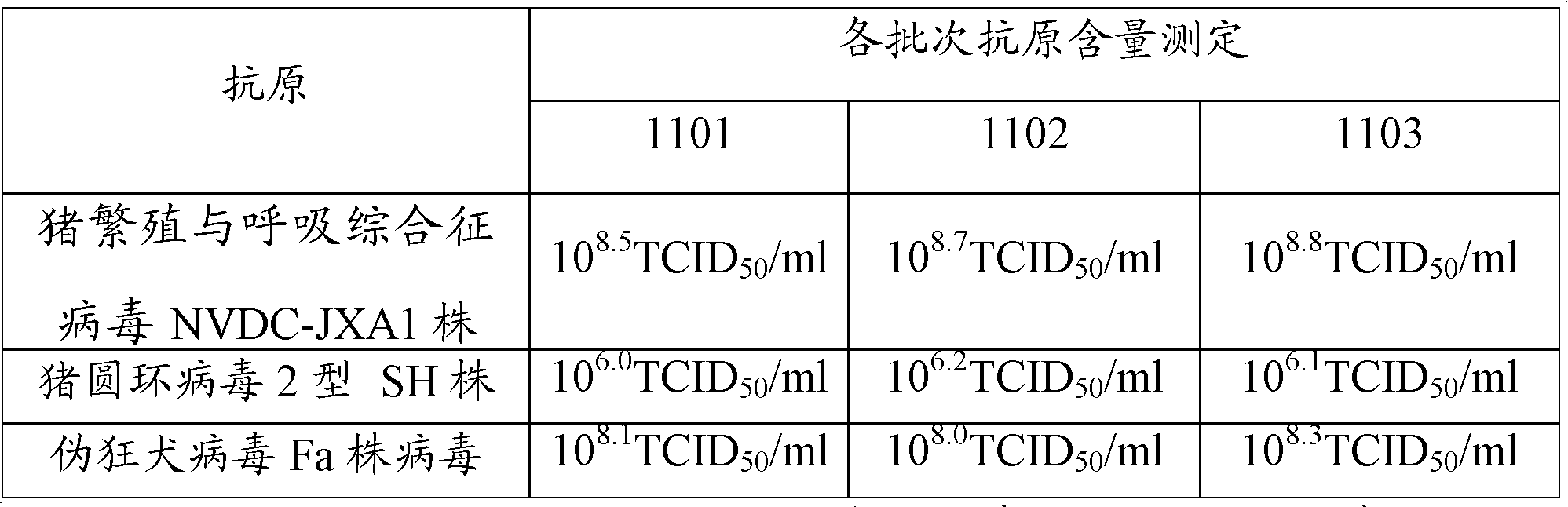
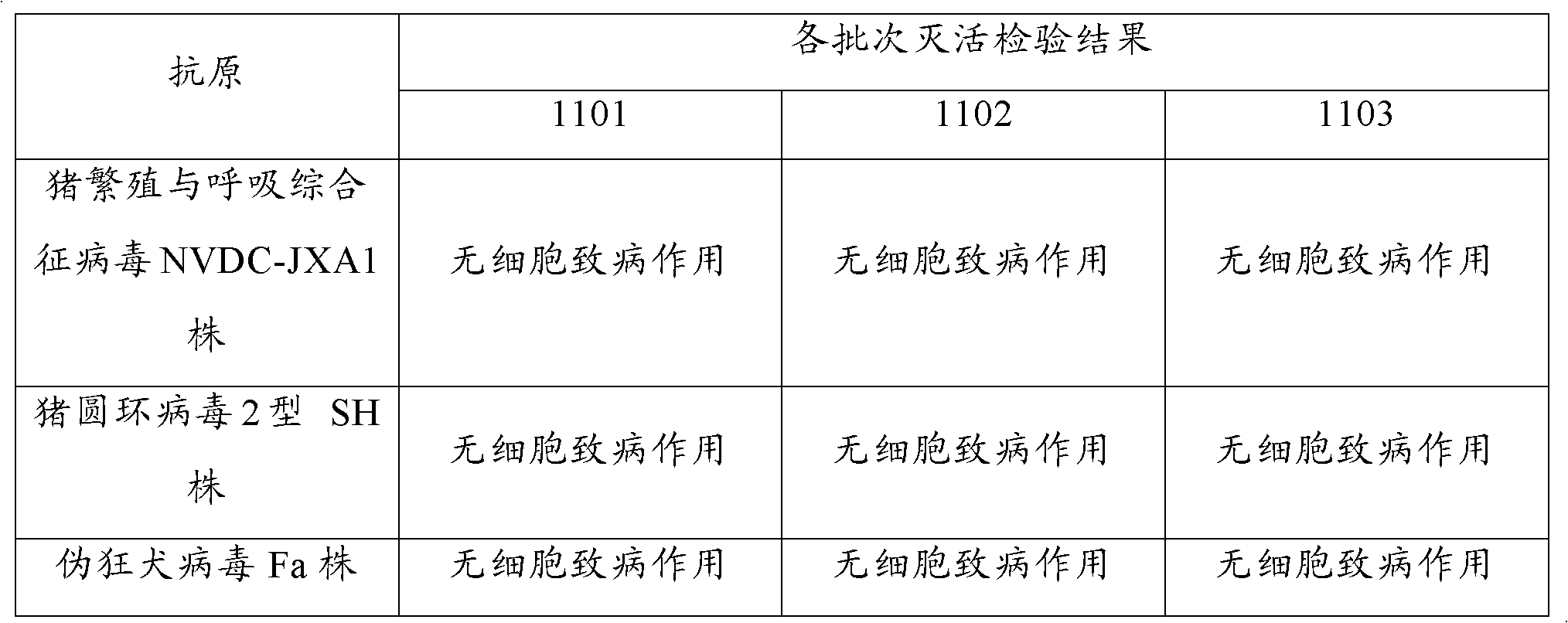
The present invention provides a trivalent inactivated vaccine of porcine reproductive and respiratory syndrome virus, porcine circovirus type 2, and porcine pseudorabies virus. Before inactivation, the contents of the three viruses are respectively greater than 10<8.5>TCID[50] / ml 10<6.0>TCID[50] / ml and 10<8.0>TCID[50] / ml; And after the inactivation, the volume ratio of the three antigens is 1:1:1. According to the present invention, via a large number of detailed tests, the contents and ratio of the three viral antigens are selected; and immune effects are measured in a large number of experimental animals and swine themselves, to ensure that the phenomenon of immune interference does not occur among the various immune components in the multivalent vaccine. Compared with the existing three individual vaccines of the same three virus, wherein three injections are need to prevent the three diseases caused by the three virus by using the three individual vaccines, the trivalent inactivated vaccine of the present invention is economical and practical, and simplifies the immunization procedure, and reduces the cost of epidemic prevention. The present invention realizes preparation and application of multivalent inactivated vaccine of porcine reproductive and respiratory syndrome virus, porcine circovirus type 2, and porcine pseudorabies virus, which has not been achieved for all the time in the field.
Owner:PU LIKE BIO ENG
Tilapia mossambica streptococcus agalactiae oral attenuated freeze-dried vaccine
InactiveCN104906568AEasy to useReduce stressAntibacterial agentsPowder deliveryStreptococcus agalactiaeStreptococcus mastitidis
The invention discloses a tilapia mossambica streptococcus agalactiae oral attenuated freeze-dried vaccine and its preparation method. According to the invention, tilapia mossambica streptococcus agalactiae attenuated bacterial strain YM001 is performed with steps of enlarge cultivation, thalline collection and freeze-drying preparation to obtain the oral attenuated vaccine. The immunizing dose, immunization program and a vaccine usage method of the vaccine can be determined through tests. The immune efficacy, security and maneuverability of the vaccine can be detected and confirmed through field tests, the result shows that the vaccine has the advantages of high protection effectiveness, good security, convenient usage and low cost, the vaccine can be used for preventing tilapia mossambica streptococcus agalactiae, and has high business value.
Owner:GUANGXI ACADEMY OF FISHERY SCI
Pseudomonas aeruginosa, klebsiella and pasteurella triple-inactivated vaccine for mink
ActiveCN104740622APrevention of mixed infectionHigh immune protection rateAntibacterial agentsAntibody medical ingredientsDiseaseMicrobiology
The invention discloses a pseudomonas aeruginosa, klebsiella and pasteurella triple-inactivated vaccine for a mink. The vaccine is composed of inactivated pseudomonas aeruginosa, klebsiella and pasteurella, and is capable of simultaneously preventing a pseudomonas aeruginosa disease, a klebsiella disease and a pasteurella disease, and mixed infection with pseudomonas aeruginosa, klebsiella disease and pasteurella disease. The vaccine is small in side effect, has synergistic effect, has relatively good immunogenicity and safety, and can reach multiple prevention effects; the immune procedure for vaccination is reduced; and the protection efficiency is improved.
Owner:JILIN HEYUAN BIOENG LIMITED
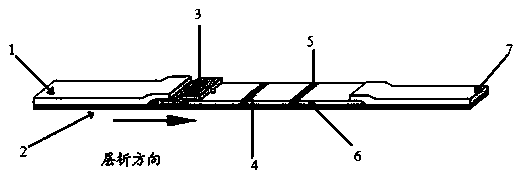
Detection card for rapidly detecting canine distemper virus antigens and preparation method of detection card
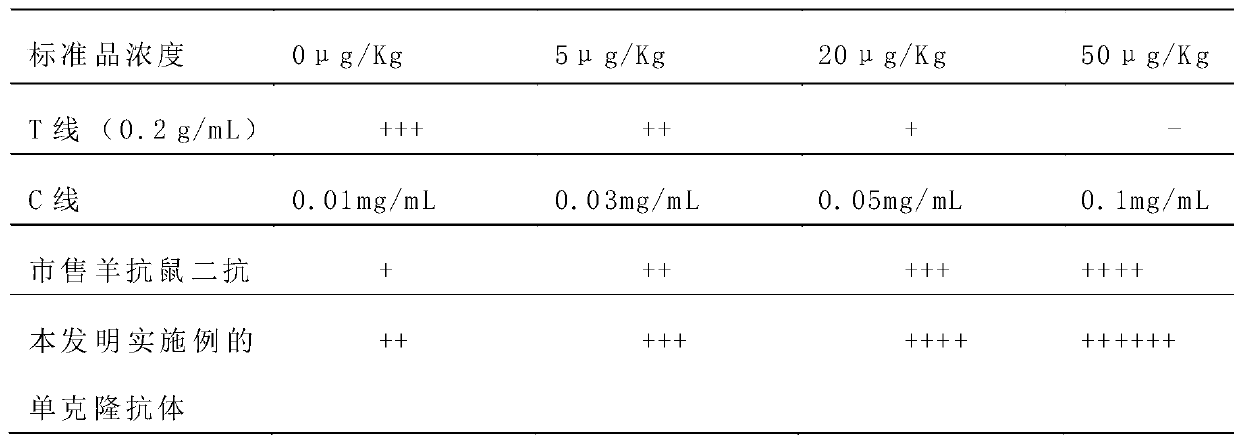
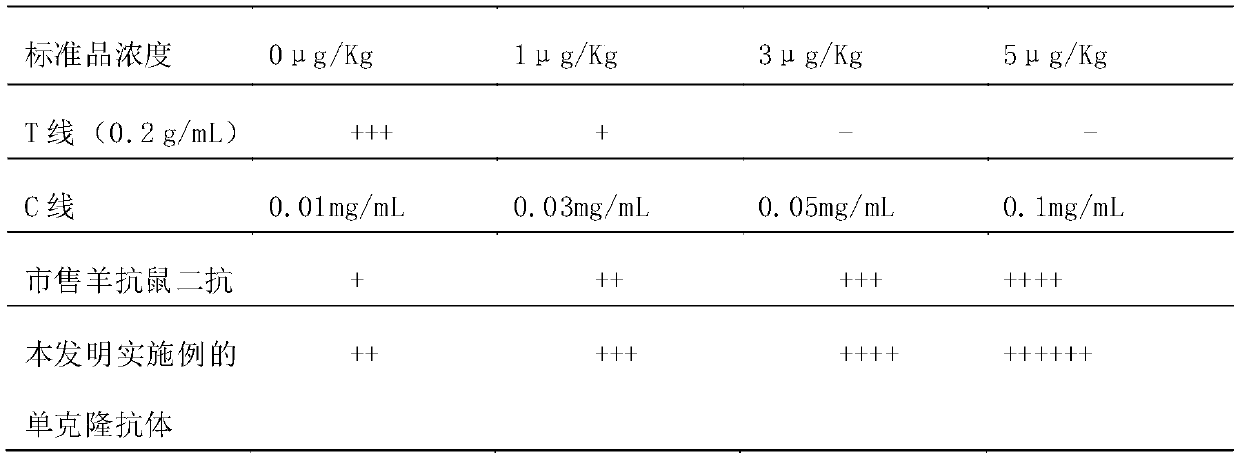
ActiveCN109975541ASolve the problem of not being able to effectively distinguish between seropositives caused by canine distemper virus infection and seropositives after vaccinationEffective controlMaterial analysisCanine distemper virus AntigenTest sample
The invention relates to a detection card for rapidly detecting canine distemper virus antigens and a preparation method of the detection card. The method includes the following specific steps of: (1)the isolation, culture and purification of canine distemper viruses; (2) the preparation of paired monoclonal antibodies against the canine distemper viruses, wherein the preparation involves animmunization procedures, cell fusion, hybridoma screening and cloning, antibody preparation and purification; and (3) the preparation method of the colloidal gold detection card for rapidly detecting the canine distemper viruses, wherein the preparation method involves sample pad treatment, film spraying, and C, T line determination, to-be-tested sample processing and test card performance measurement.With the preparation method of the detection card of the invention adopted, canine distemper viruses in China can be quickly, sensitively and accurately detected. According to the prior art, paired monoclonal antibodies for developing colloidal gold detection cards for detecting canine distemper viruses in China are made of imported raw materials; the imported raw materials of the monoclonal antibodies are prepared from current viruses isolated by a certain country. With the preparation method of the invention adopted, the conditions in the prior art can be avoided, and a phenomenon that missed detection caused by virus variation due to long distances between areas during a detection process can be avoided.
Owner:SHANDONG LVDU BIO SICIENCE & TECH
Vaccine composition resisting porcine circovirus and porcine infectious pleuropneumonia infection and preparation
ActiveCN103656634AHigh antigen titerImmunization is convenient and fastAntibacterial agentsAntiviralsDiseaseImmune effects
The invention relates to a bivalent vaccine composition resisting type-2 porcine circovirus and porcine infectious pleuropneumonia infection and a preparation method. The bivalent vaccine composition comprises a type-2 porcine circovirus antigen and an actinobacillus pleuropneumoniae antigen; the immune procedure is simple; the type-2 porcine circovirus related diseases and porcine infectious pleuropneumonia can be effectively prevented at the same time. Compared with a single vaccine, the bivalent vaccine composition does not generate an immune interference phenomenon while the immune effect is not reduced; moreover, the actinobacillus pleuropneumoniae can enhance the immune effect of the type-2 porcine circovirus antigen. The preparation process of the bivalent vaccine composition is simple, the immune cost is low, and the practicability is high.
Owner:PU LIKE BIO ENG
Preparation method and application of micro-capsule coated egg yolk antibody IgY resistant to main pathogenic bacteria of dairy cow mastitis
InactiveCN106496325AFully emulsifiedGood immune effectAntibacterial agentsEgg immunoglobulinsEscherichia coliSolubility
The invention discloses a preparation method and application of a micro-capsule coated egg yolk antibody IgY resistant to main pathogenic bacteria of dairy cow mastitis. A compound mycoprotein antigen is prepared by selecting streptococcus agalactiae, streptococcus dysgalactiae, staphylococcus aureus and escherichia coli and is mixed with an equal quantity of freund's adjuvant to prepare a multivalence antigen immune complex, laying hens are immune by adopting repeatedly alternative immune procedures and a muscular and subcutaneous multipoint injection means, a specific yolk immunoglobulin IgY resistant to the main pathogenic bacteria of dairy cow mastitis, namely DP-BM-IgY, is separated and purified from collected egg yolk, then homogeneous emulsification is conducted on the specific yolk immunoglobulin and a sodium alginate water solution and an emulsifying agent soya bean salad oil containing span-80, the emulsified solution is dropwise added into an encystation solution prepared from CaCl2 and chitosan for coating and curing, and the coated micro-capsules can effectively protect the activity of the DP-BM-IgY and have enteric solubility. The preparation method utilizes a micro-capsule technology and selects the chitosan and sodium alginate as coating materials (natural polysaccharides), and the egg yolk antibody is internally taken and free of any toxic or side effect.
Owner:LANZHOU INST OF ANIMAL SCI & VETERINARY PHARMA OF CAAS
Antibody joint detection kit containing porcine pseudorabies virus gD and gE proteins and preparation method and application thereof
PendingCN113671182AImprove operation convenienceImprove operational accuracyBiological material analysisRabiesEngineering
The invention relates to an antibody joint detection kit containing porcine pseudorabies virus gD and gE protein antibodies. The joint detection kit comprises one or more porcine pseudorabies virus gD and gE protein antibody detection chips and an enzyme-labeled reagent, the detection chips are coated with porcine pseudorabies virus gD protein, gE protein, a quality control product and a blank control point, the enzyme-labeled reagent is a solution containing an enzyme-labeled porcine pseudorabies virus gD and gE protein monoclonal antibody, the quality control product is a goat anti-mouse polyclonal antibody, and the coating amount is 1-4 ng / dot. According to the kit prepared by the invention, the quality control product is arranged on the reaction carrier, negative control does not need to be arranged in the kit, the detection is rapid, the operation is simple and convenient, the integration is high, one-button intelligent data processing is realized, the kit can be used for detecting the infection state of the porcine pseudorabies virus in a swinery, evaluating the immune effect of the porcine pseudorabies virus vaccine in the swinery and identifying the infection condition of the newly introduced porcine pseudorabies virus, and is convenient for formulating an immune program and carrying out early purification.
Owner:洛阳中科生物芯片技术有限公司
Preparation method of genetic engineering vaccine for preventing staphylococcus caprae mastitis application
ActiveCN102847172AImprove immunityEasy to makeAntibacterial agentsGenetic material ingredientsDiseaseMastitis
The invention belongs to the technical field of animal genetic engineering, and particularly relates to a preparation method of a genetic engineering vaccine for preventing staphylococcus caprae mastitis application. The preparation method comprises the following steps: 1) amplifying target genes ClfA, ClfA and Sbi; 2) building a Pci-Hla / ClfA-A / Sbi-I-II recombinant eukaryotic expression vector; and 3) establishing an immune procedure, and evaluating the immune result. According to the preparation method provided by the invention, the genetic engineering vaccine is on the basis of rapidly developed molecular biological technique, hereditary and immunology, and comprises a genetic subunit vaccine, a genetic mutation vaccine and a nucleic acid vaccine, wherein the nucleic acid vaccine is a DNA (Deoxyribose Nucleic Acid) vaccine, is an eukaryotic expression vector for to recombining and building one or more antigen-encoding genes, and can be directly injected into an organism to activate an immune system, so as to achieve the purpose of preventing and treating disease.
Owner:NORTHWEST A & F UNIV
Method for cleansing epidemic diseases on scale pig farm
The invention relates to the technical field of epidemic disease immunization on pig farms, in particular to a method for cleansing epidemic diseases on a scale pig farm. The method comprises the following steps: sampling serum samples of boars in phases and sampling serum samples of piglets according age in days; detecting an immune antibody and an infection antibody; revising an immune process according to results. By adopting the method, the problems involved in prevention and control, particularly immediate cleansing of major epidemic diseases on the scale pig farm are solved, the morbidity and mortality of pig diseases on the scale pig farm are controlled, the immune effect is improved, and the economic benefit is increased.
Owner:唐山市动物疫病预防控制中心
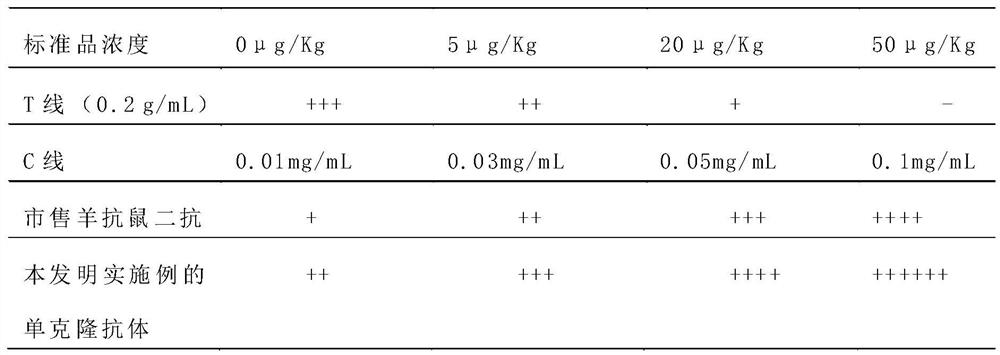
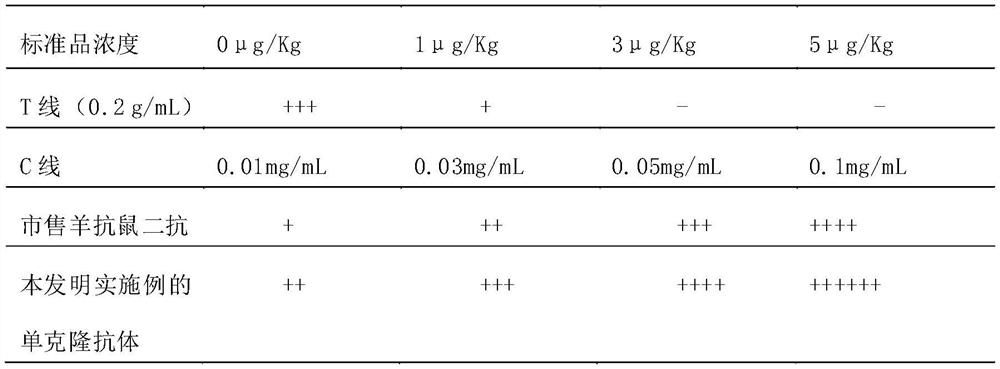
Preparation method of monoclonal antibody for replacing anti-mouse rabbit secondary antibody
ActiveCN110981963AEasy to prepareImprove stabilityImmunoglobulinsMaterial analysisAntiendomysial antibodiesMonoclonal
The embodiment of the invention discloses a preparation method of a monoclonal antibody for replacing an anti-mouse rabbit secondary antibody. The method comprises the steps of using purified rabbit serum as a holoantigen, and carrying out immunoreaction and cell fusion screening to obtain hybridoma cells. The embodiment of the invention provides the preparation method of a mouse non-specific monoclonal antibody, which uses the rabbit serum holoantigen as an immunogen, and obtains the anti-mouse rabbit secondary antibody used for replacing the prior art through immunogen purification, immune procedure, cell fusion, hybridoma screening and cloning, and antibody preparation and purification. The monoclonal antibody prepared by the embodiment of the invention is simple in preparation method,can be produced in batches, is good in stability, can be applied to immunochromatographic detection, is high in titer and strong in reaction, and is suitable for immunochromatographic reactions in various modes.
Owner:SHANDONG LVDU BIO SICIENCE & TECH
Vaccine composition for resisting pig mycoplasma pneumonia and infectious pleuropneumonia and preparation method
ActiveCN103623400AReduce harmSimplified immunization programAntibacterial agentsBacterial antigen ingredientsTGE VACCINEMycoplasma pneumonia
The invention relates to a vaccine composition for resisting pig mycoplasma pneumonia and infectious pleuropneumonia and a preparation method. The vaccine composition comprises pig mycoplasma hyopneumoniae antigens and pig actinobacillus pleuropneumoniae antigens, is simple in immunization procedure, not only is capable of generating antibodies, but also has the functions of toxicity attack protection and effectively controlling pig mycoplasma pneumonia and infectious pleuropneumonia. The vaccine composition has the immunization effect same to an independently injected vaccine, is small in side reaction, long in immunization period, less in consumed time, less in consumed labor, small in encroaching on a pig. The vaccine composition is simple in production technology, low in immunization cost and strong in practicality.
Owner:PU LIKE BIO ENG
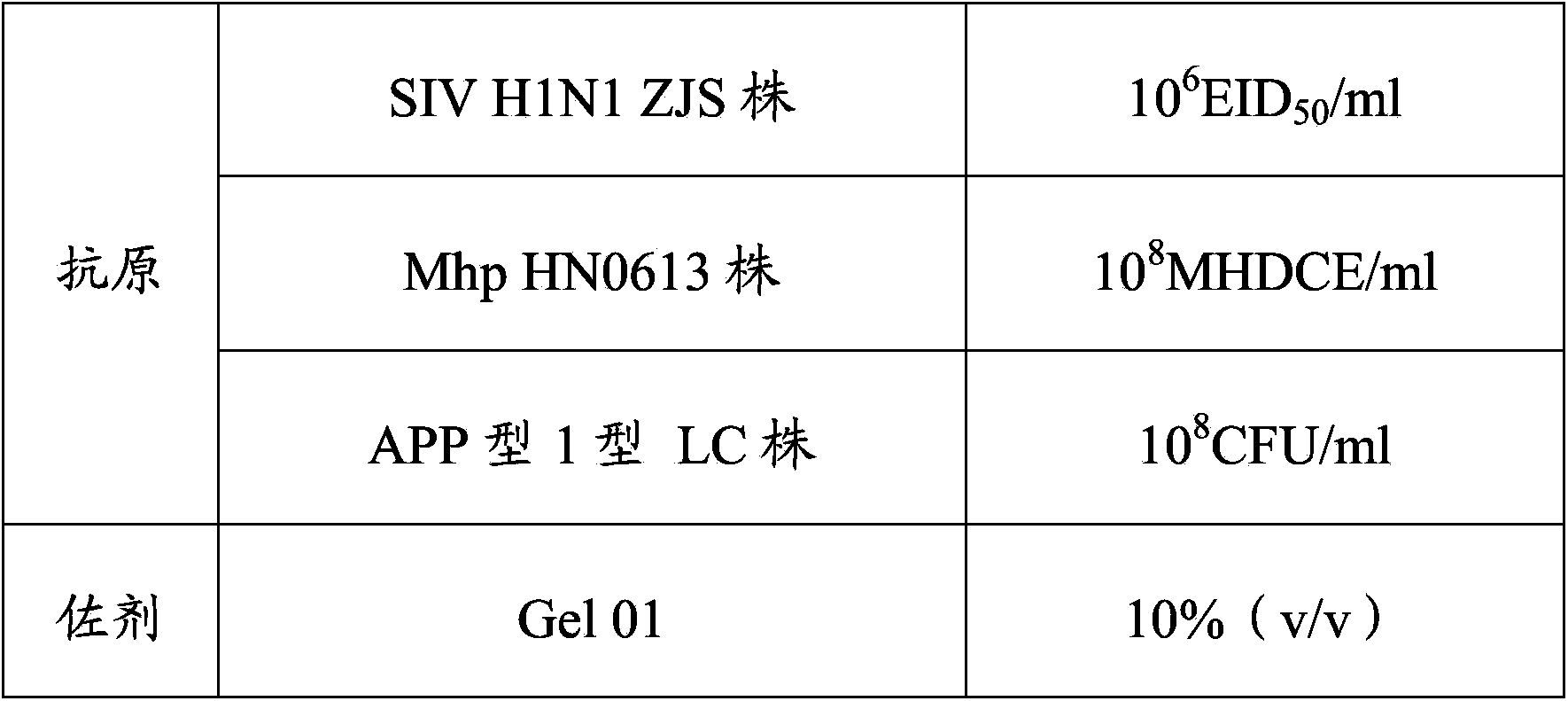
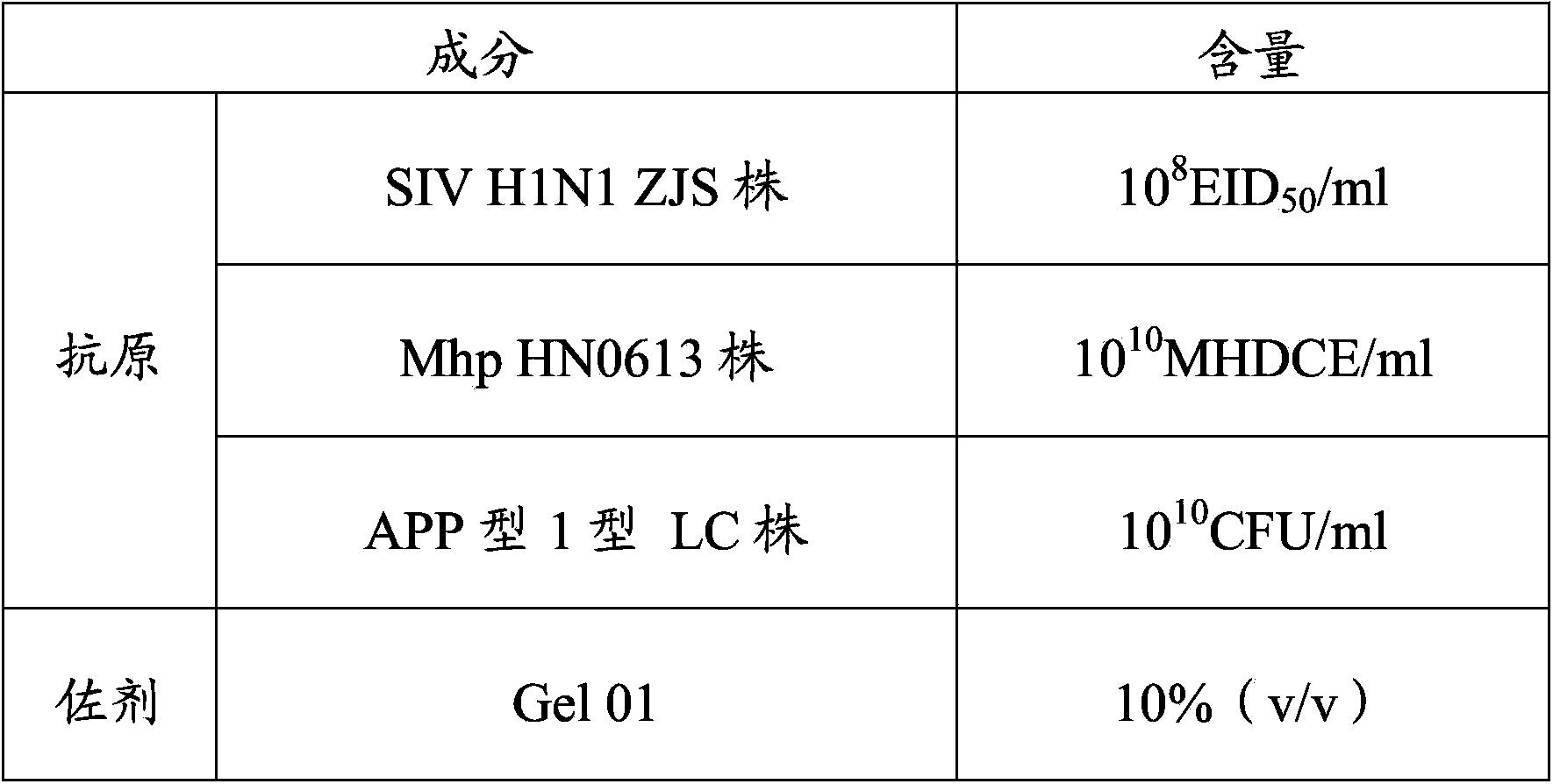
Vaccine composition and preparation method and application thereof
The invention provides a vaccine composition. The vaccine composition comprises SIV antigens in immune amount, Mhp antigens in immune amount, APP antigens in immune amount and a carrier acceptable on the veterinary medicine. The vaccine composition is simple in immune procedure; and single infecting or mixed infecting of SIV, Mhp and APP can be effectively prevented and treated. When mixed infecting occurs, the immune effect of the vaccine composition is obviously better than the immune effect of respectively-injected single vaccines. The vaccine composition is small in composition side reaction, long in immunity period, short in spent time, less in wasted labor, simple in production process, low in immune cost and high in practicability.
Owner:PU LIKE BIO ENG
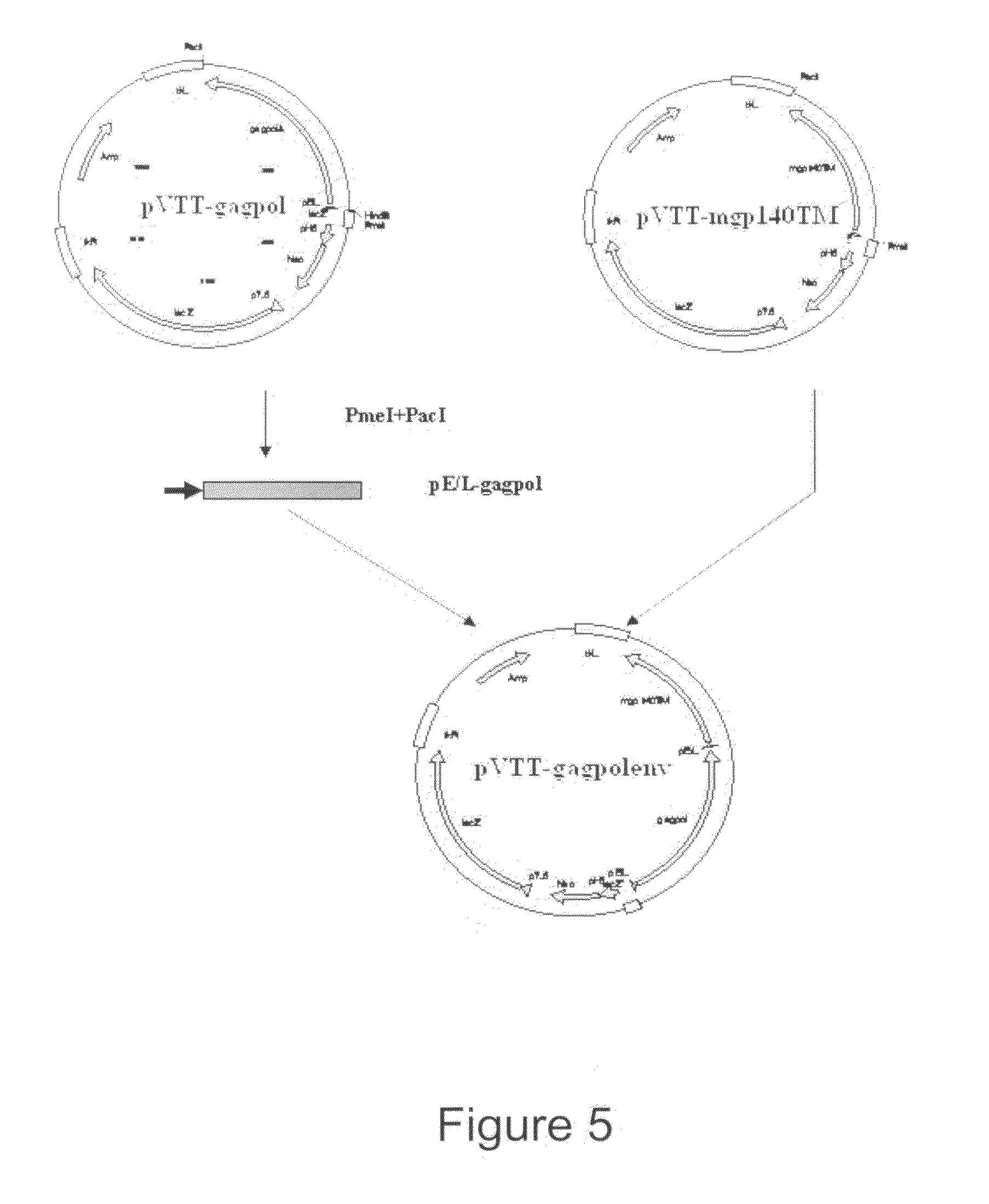
AIDS Vaccine Based on Replicative Vaccinia Virus Vector
Replicative live-vector vaccine against AIDS expressing antigen of human immunodeficiency virus (HIV) and its application are provided. Said vaccine is constructed on the basis of replicative vaccinia virus, such as vaccinia virus TianTan strain. The replicative live-vector vaccine of present invention is capable of inducing high level humoral and cell immunoresponse against HIV. The vector for constructing said vaccine, and immunization procedure using said vaccine are also provided.
Owner:NAT CENT FOR AIDSSTD CONTROL & PREVENTION CHINESE CENT FOR DISEASE CONTROL & PREVENTION
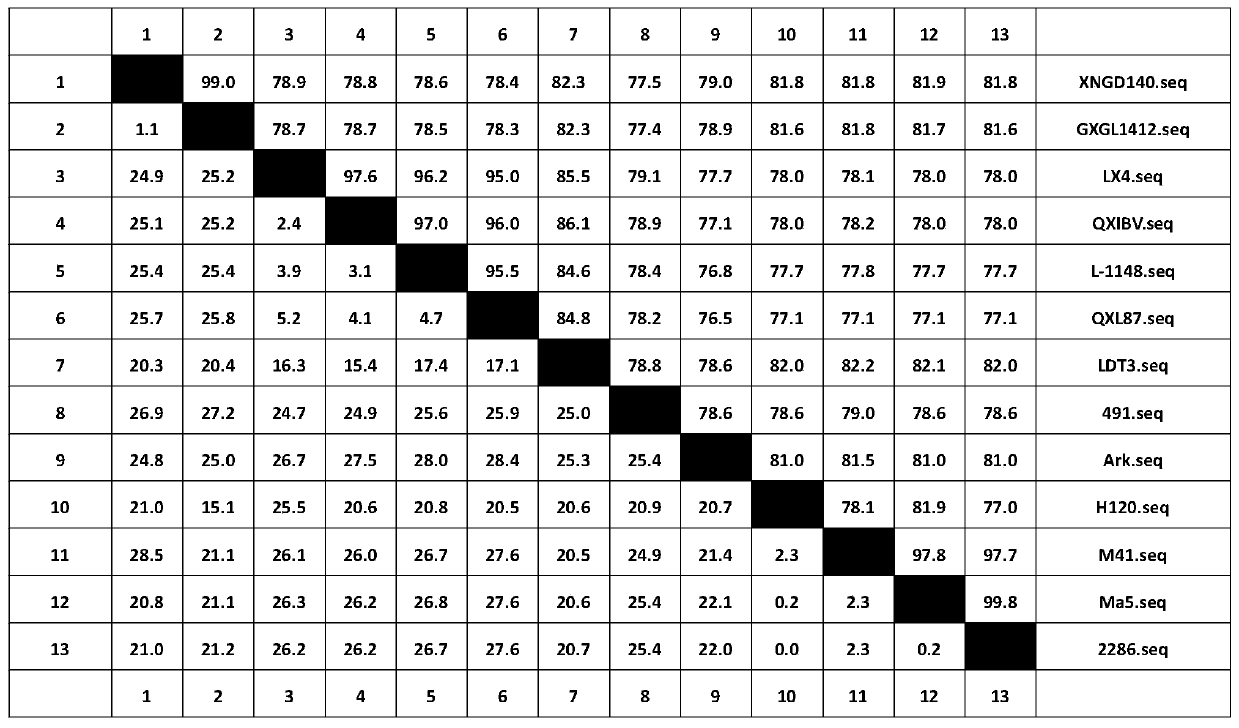
Method for preparing TW1 type avian infectious bronchitis virus positive serum from SPF chickens
ActiveCN110467671AImprove the level of prevention and controlSolve the current situation of no standard positive serumSerum immunoglobulinsImmunoglobulins against virusesSerum igeGenotype
The invention discloses a method for preparing TW1 type avian infectious bronchitis virus positive serum from SPF chickens. An IBV virus seed of a specific genotype (TW1 type) is used. The method comprises the steps: the IBV CK / CH / XNGD / 140 strain virus seed and an oil emulsion inactivated vaccine thereof are used for conducting basic immunization and enhanced immunization on SPF chickens which are50 days old, blood sampling is conducted, and serum is separated; and the TW1 type IBV positive serum with good specificity and high titer is obtained by carrying out sterile and exogenous virus detection, neutralization titer determination and specificity detection on the serum. The preparation method of the TW1 type IBV positive serum fills the blank that the TW1 type IBV virus seed has no standard positive serum. The SPF chickens are selected as test animals for preparing the serum, the immune procedure in the serum preparation process is optimized, and the serum antibody neutralization titer is improved. The production cost is reduced, and batch preparation of the positive serum is facilitated.
Owner:ZHONGCHONG XINNUO BIOTECH TAIZHOU CO LTD
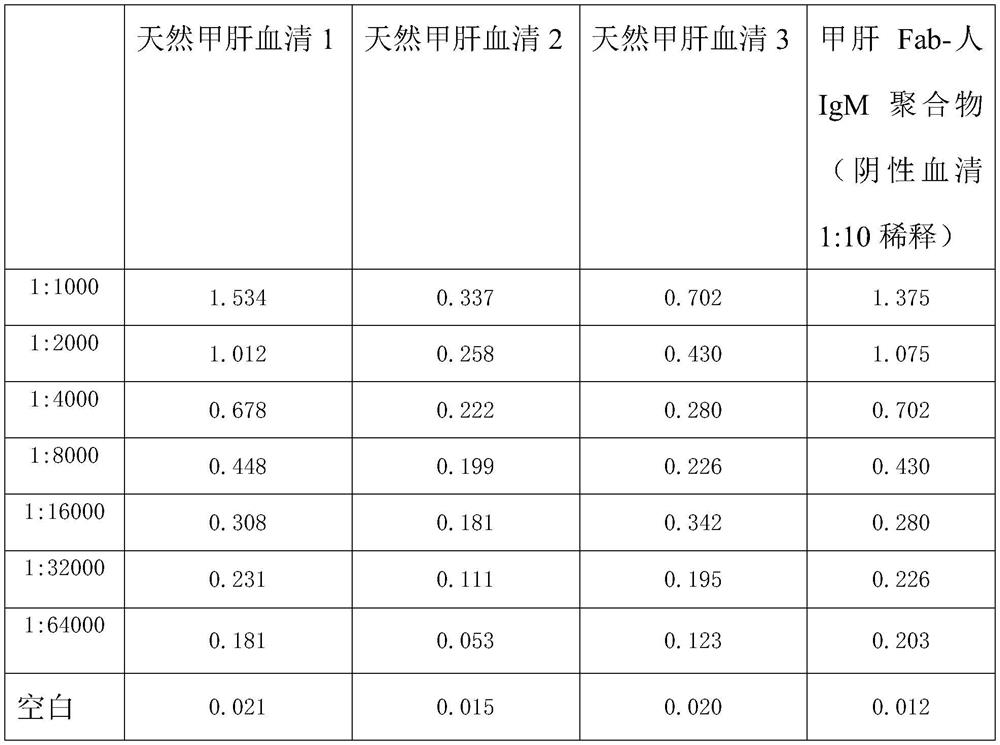
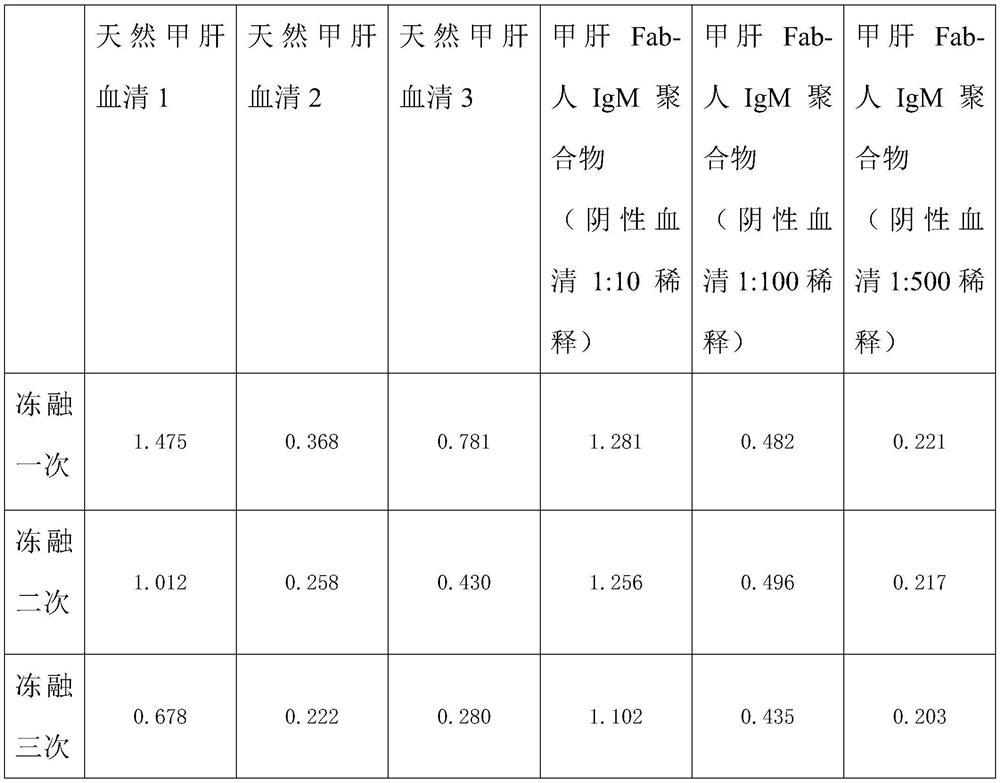
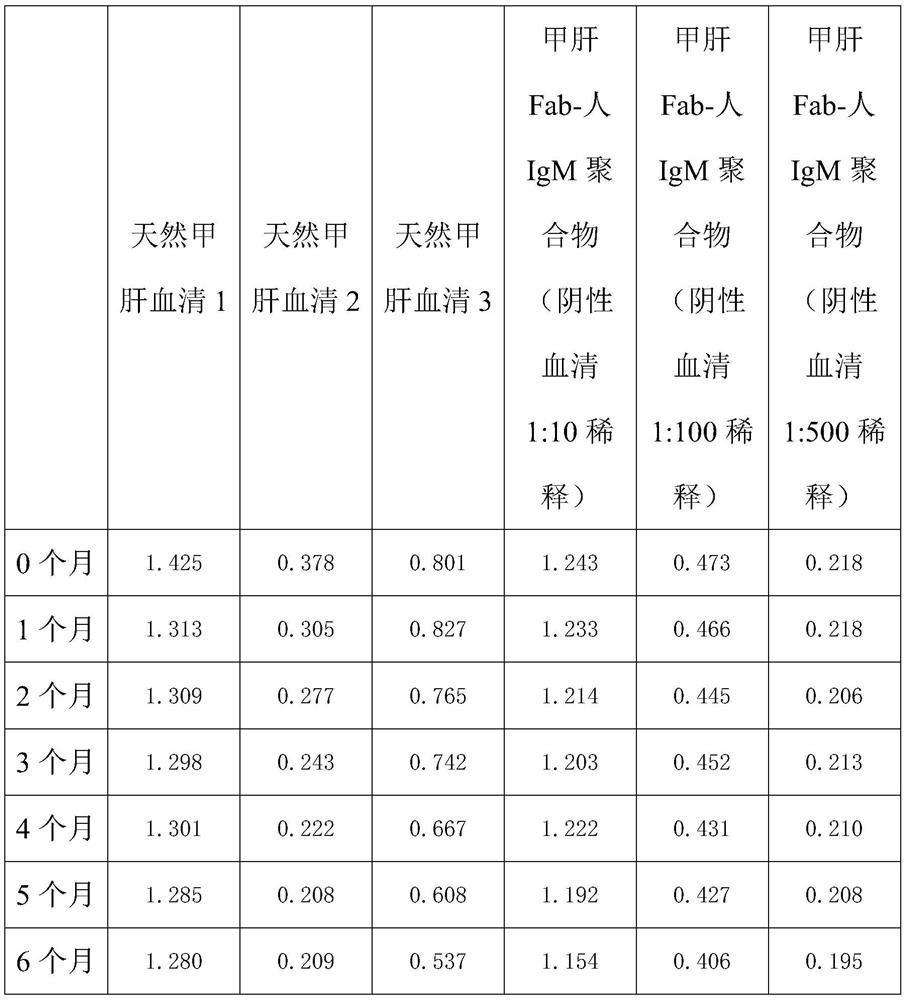
Method for preparing IgM (Immunoglobulin M) calibrator
PendingCN114544295AHigh affinityImprove stabilityPreparing sample for investigationIgm antibodyAntiserum
The invention discloses a method for preparing an IgM calibrator, and relates to the field of IgM calibrators, the method comprises the following steps: 1, obtaining a sheep polyclonal antibody: immunizing a sheep according to a fixed immunization program, and extracting serum when the titer is qualified; 2, purifying the sheep polyclonal antibody: purifying the extracted sheep polyclonal antibody serum by using affinity chromatography; 3, cutting the sheep polyclonal antibody: cutting the sheep polyclonal antibody IgG by using an enzyme, purifying a Fab fragment in the sheep polyclonal antibody, and using the purified Fab fragment for subsequent coupling; 4, coupling of the human IgM and the Fab fragment: coupling the human IgM and the Fab fragment of the sheep polyclonal antibody by using a chemical method; 5, detection: carrying out affinity test and aging test on a product obtained by coupling the human IgM and the Fab fragment of the sheep polyclonal antibody; the prepared specific IgM is added into negative serum according to a certain concentration to obtain the IgM antibody serum, and the IgM antibody serum can be used for research and development of in-vitro diagnosis IgM kits and calibration products of the specific IgM serum.
Owner:武汉原谷生物科技有限责任公司
Tumor vaccine treatment method
InactiveCN109833473AWide variety of sourcesEasy accessAntibody medical ingredientsAntineoplastic agentsAntigenCytokine
The invention discloses a tumor vaccine treatment method, which comprises the following steps: patient preparation, preoperative examination, informed agreement, tumor tissue resection, postoperativetreatment, gp96 protein purification and clinical treatment. According to an immunization procedure, the patient is subjected to administered subcutaneous or intradermal injection with a tumor autologous gp96 vaccine, and gp96 interacts with heat shock protein receptors (CD91, CD36, SR-A, Lox-1, TLR2 and TLR4) on the surface of antigen presenting cells, the maturation and activation of antigen-presenting cells can be promoted, which are realized by up-regulating of cell surface molecules and secreting of cytokines and chemokines, the adjuvant properties of gp96 and its bonded tumor-derived polypeptides provide an ideal environment for cross-presenting MIHC I and MHC II restricted polypeptides, tumor autologous gp96 activates tumor cells and micrometastases of tumor cell-specific CD4<+> helper T cells and cytotoxic CD8<+> T cells, the method has good immunization characteristic, and the treatment effect is better than that of the operation and chemotherapy.
Owner:杨涛
Vaccine composition containing swine mycoplasmal pneumonia antigen and swine streptococcosis antigen, and preparation method and application thereof
ActiveCN103861095APreserve immune efficiencyLow costAntibacterial agentsBacterial antigen ingredientsImmune effectsAdjuvant
The invention provides a vaccine composition containing a swine mycoplasmal pneumonia antigen and a swine streptococcosis antigen, and a preparation method and an application thereof. The vaccine composition includes an immunizing dose of the swine mycoplasmal pneumonia antigen, an immunizing dose of the swine streptococcosis antigen, and an adjuvant. The vaccine composition has a simple immunization program, can effectively control the swine mycoplasmal pneumonia antigen and the swine streptococcosis antigen, has an immune effect equivalent to the immune effect realized through respective injection of single vaccines, and also has the characteristics of small side reaction, long immune period, less time consumption, and less labor consumption; and the vaccine composition also has the advantages of simple production technology, low immune cost and strong practicality.
Owner:PU LIKE BIO ENG
Porcine circovirus, porcine pseudorabies virus and mycoplasma triple inactivated vaccine
PendingCN112957460AReduce the chance of side effectsHigh antigen contentAntibacterial agentsBacterial antigen ingredientsUltrafiltrationVirus Protein
The invention discloses a porcine circovirus, porcine pseudorabies virus and mycoplasma triple inactivated vaccine which comprises an antigen and a vaccine adjuvant, the antigen is composed of a porcine circovirus type 2 antigen, a porcine pseudorabies virus antigen and a mycoplasma antigen, the porcine circovirus type 2 antigen is a purified, concentrated and inactivated porcine circovirus type 2 protein antigen solution, and the content of Cap protein is more than or equal to 160 [mu]g / ml; the porcine pseudorabies virus antigen is a purified, concentrated and inactivated porcine pseudorabies virus protein antigen solution, and the content of the Cap protein is more than or equal to 160 [mu]g / ml; the mycoplasma antigen is an inactivated mycoplasma protein antigen solution, and the content of the Cap protein is more than or equal to 160 [mu]g / ml; and the vaccine adjuvant is composed of a water-based high-molecular polymer adjuvant and a composite polysaccharide immunopotentiator. Foreign protein is removed through clarification filtration and ultrafiltration concentration, and the side reaction probability of the vaccine is greatly reduced; and three-proofing can be achieved through one needle, so that the number of immunization times and stress are reduced. The method is economical and practical, the immunization procedure is simplified, and the epidemic prevention cost is reduced.
Owner:JIANGXI ZHENGBANG TECHNOLOGY CO LTD +1
Treatment method of human peripheral blood T lymphocytes, immunogen preparation and application
ActiveCN113025571ASolve bottlenecksSimplify the production purification processSerum immunoglobulinsImmunoglobulins against animals/humansWhite blood cellT lymphocyte
The invention relates to a treatment method of human peripheral blood T lymphocyte, animmunogen preparation, a preparation method of antiserum, and application. T lymphocytes are separated and purified by using a waste leukocyte filter disc, multiplication culture is carried out, and antigen surface marker identification comparison and activity comparison are carried out on the separated T lymphocytes and the cultured T lymphocytes; the high-activity T lymphocytes subjected to multiplication culture are used as an immunogen, antiserum is prepared by using a healthy pig, the titer after immunization is evaluated by using results of an E rose ring inhibition experiment and a cytotoxicity experiment, and formulating an immunization program, so as to prepare a large amount of antiserum; and anti-plasma is collected for plasma separation, and the plasma separation is used for preparation of ALG products. According to the invention, the limitation problem that fresh whole blood needs to be collected every time in the titer verification of the anti-human T lymphocyte immune globulin preparation raw material and the titer verification of the finished product can be solved, and meanwhile, the bottleneck problem of the anti-human T lymphocyte immune globulin raw material can also be solved.
Owner:武汉中生毓晋生物医药有限责任公司
A preparation method of monoclonal antibody used to replace anti-pika secondary antibody
ActiveCN110981963BEasy to prepareImprove stabilityImmunoglobulinsMaterial analysisAntiendomysial antibodiesBiochemistry
The embodiment of the present invention discloses a preparation method of a monoclonal antibody used to replace the anti-pika secondary antibody. The method includes using purified rabbit serum as the whole antigen, and obtaining hybridoma cells through immune reaction and cell fusion screening. process. The embodiment of the present invention provides a method for preparing a mouse non-specific monoclonal antibody, which uses the whole rabbit serum antigen as an immunogen, through immunogen purification, immunization procedures, cell fusion, hybridoma screening and cloning, antibody preparation and purification Obtained and used to replace the anti-pika secondary antibody used in the prior art. The monoclonal antibody prepared in the embodiment of the present invention has a simple preparation method, can be produced in batches, and has good stability. It can be applied to immunochromatographic detection, has high titer and strong reaction, and is suitable for various modes of immunochromatographic reactions.
Owner:SHANDONG LVDU BIO SICIENCE & TECH
Composition, Preparation Method And Evaluation Of A Complex Immunogen Named I-SPGA For Production Of Immunological Active Proteins (IAP)
ActiveUS20210121552A1Increased antibody coupling powerEasy to combineBacterial antigen ingredientsEgg immunoglobulinsPotentiatorResistant infection
The present invention relates to the composition and method of preparing an immunogen designated as I-spga consisting of a complex antigen prepared from 18 to 26 species of pathogenic microorganisms isolated from patients, inactivated with binary ethyleneamine (BEI) and formalin, diluted in a SPGA immunopotentiator mixed with QS-21 adjuvant. By inoculating the hens with the I-spga immunogen, hyperimmune eggs (Immunospga) are obtained which contain immunologically active proteins specific to the 18-26 antigens used for immunization. The immune response of the hens is specific to the used antigens by amplification of the antigenic signal by the SPGA immunopotentiator and due to a special immunization program that allows the immune system to act complex and intense: The I-spga complex antigen contains 18-26 microorganisms isolated from patients, bacterial bodies, components from bodies obtained by ultrasonography, cilia, exotoxins, endotoxins, spores, viruses, fungi or yeasts. This pathogenic material is inactivated with BEI and formalin. The I-spga antigen is of three types. The standard I-spga antigen is composed of 18 to 24 antibiotic-resistant bacterial species isolated from patients in Romania. The specific I-spga complex antigen is composed of the I-spga complex antigen containing a mixture of 7-9 strains from a single species of bacteria, fungi or yeasts isolated from patients in Romania mixed with SPGA and QS-21, used for inoculation of hens previously immunized with standard I-spga antigen. The personalized I-spga antigen is composed of patient-derived pathological material containing cellular debris and pathogenic germs inactivated with BEI and formalin and mixed with SPGA and QS-21 and is used to immunize hens previously immunized with the standard I-spga antigen. This now patented technology encompasses a new generation of biological products in which the immune response of the hens to different groups of parenterally inoculated antigens at different time intervals is overlapping. Chicken response is uniform and additional administration of immunogens and SPGA as an immunopotentiator amplifies the antigenic signal and immune response. The I-spga immunogen as well as the immune response contain two markers, G and A, which identify the I-spga antigen used for immunization against the antigens used to produce the Imunoinstant group bio-preparations or similar products. The I-spga immunogen is used to immunize the hens for obtaining immunologically active proteins that can be used to treat immune deficiencies, psoriasis, epidermolysis bullosa, other dermatitises, nosocomial infections, antibiotic-resistant infections in the urinary system of children and grownups.
Owner:FANTANA RAUL SORIN +1
Monoclonal antibody for detecting new coronavirus
PendingCN113754761AEfficient detectionImprove accuracyBiological material analysisImmunoglobulins against virusesAffinity purified antibodyIncreased igm
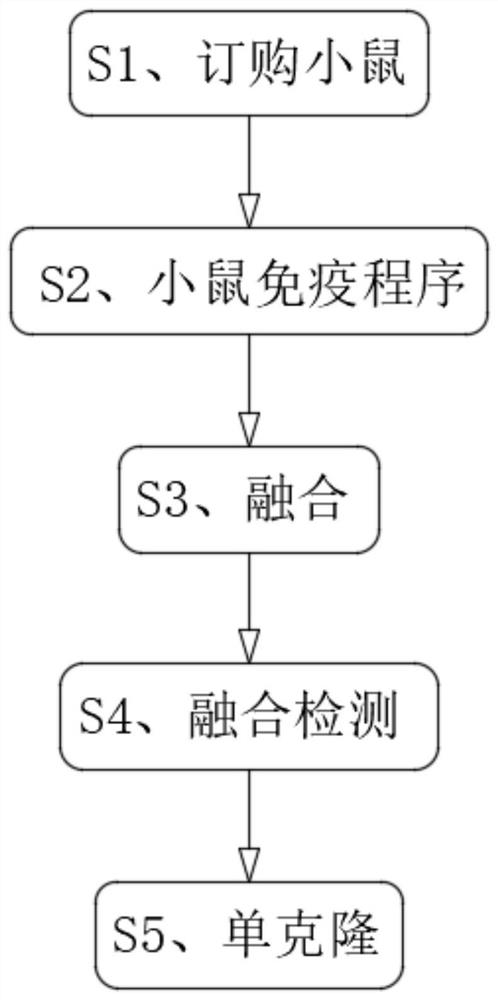
The invention discloses a monoclonal antibody for detecting a new coronavirus. The formula of the monoclonal antibody for detecting the new coronavirus comprises the following components by weight: 1-3mg of antibody purified by protein G for ascetic fluid preparation, 0.5-1.5 mg of an antibody purified by antigen for ascetic fluid preparation, 1-3mg of an antibody purified by ammonium sulfate precipitation for ascetic fluid preparation, 1-3mg of an antibody purified by protein G for cell supernatant enlarge cultivation, 0.5-1.5 mg of an antibody purified by antigen for cell supernatant enlarge cultivation; the monoclonal antibody for detecting the new coronavirus comprises the following steps of S1, mouse ordering, S2, mouse immunization procedure, S3, fusion, S4, fusion detection and S5, monoclone; the monoclonal antibody for detecting the new coronavirus can effectively detect the new coronavirus, solves the problem of easy missed diagnosis in nucleic acid detection, increases IgM and IgG antibody detection when the nucleic acid detection is negative, can be used as a supplementary means for nucleic acid diagnosis, and greatly improves the accuracy of the new coronavirus detection.
Owner:苏州博特龙免疫技术有限公司
Novel flow method adeno-quadruplet virus-like particle and preparation method and application thereof
ActiveCN112458118BSimplified immunization programAvoid mutual interferenceSsRNA viruses negative-senseViral antigen ingredientsVirus-like particleViral nucleic acid
A new flow method adeno-quadruplet virus-like particle and its preparation method and application belong to the field of quadruple vaccine research and development. Rescue, preparation and purification of chimeric virus-like particles, and finally obtain chimeric virus-like particles NDV‑AIV‑IBDV‑FAdV4 cVLPs. The new flow method AAV-like particle prepared by the preparation method of the present invention has the following advantages: the new flow method AAV-like particle can repeatedly display foreign antigens with high density, and has strong immunogenicity; It does not contain viral nucleic acid, green and safe, which is conducive to the purification of new flow glands, and conforms to the modern green and safe breeding concept; the vaccine seed virus is matched with the popular virus strain; one shot for multiple prevention, simplifying the vaccine immunization procedure, avoiding a variety of Mutual interference of vaccines; suitable for large-scale suspension culture and mass production.
Owner:JILIN UNIV
High-titer hepatitis B immunized plasma and preparing process thereof
InactiveCN102580089ASimple preparation processStrong maneuverabilityAntiviralsAntibody ingredientsViral hepatitis bHepatitis B immunization
The invention relates to high-titer hepatitis B immunized plasma and a preparing process of the immunized plasma. The titer of a hepatitis B antibody is more than 8IU / ml. The preparing process comprises the following steps of: (1) grouping: dividing blood donors with HBsAb (hepatitis B surface antibody) less than 10mIU / ml and more than 10mIU / ml into two groups, and immunizing each group according to different immunizing procedures and different immunizing doses; (2) raw material plasma collecting manner: collecting by adopting a full-automatic plasma collector; (3) detection method: performing operation according to the specification of an HBsAb kit by adopting ELISA (enzyme-linked immunosorbent assay); and (4) judgment standard: judging the detection result HBsAb more than 10mIU / ml to be antibody positive conversion and the plasma with HBsAb more than 8IU / mL to be high-titer HBsAb plasma. According to the invention, the titer of the hepatitis B antibody in the high-titer hepatitis B immunized plasma is more than 8IU / ml; and the preparing process is simple and strong in operability.
Owner:HUNAN KANGRUN PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com