Vaccine composition resisting porcine circovirus and porcine infectious pleuropneumonia infection and preparation
A technology of porcine circovirus and porcine pleuropneumonia, applied in antiviral agents, antibacterial drugs, antibody medical components, etc., can solve the problems of immune stress response and immune effect reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
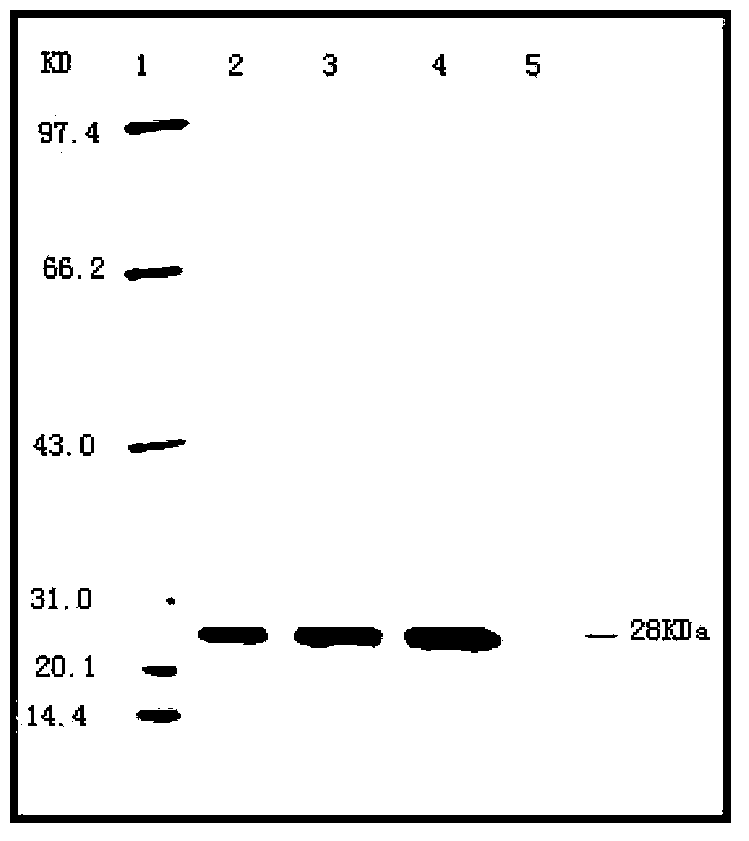
Image
Examples
Embodiment 1
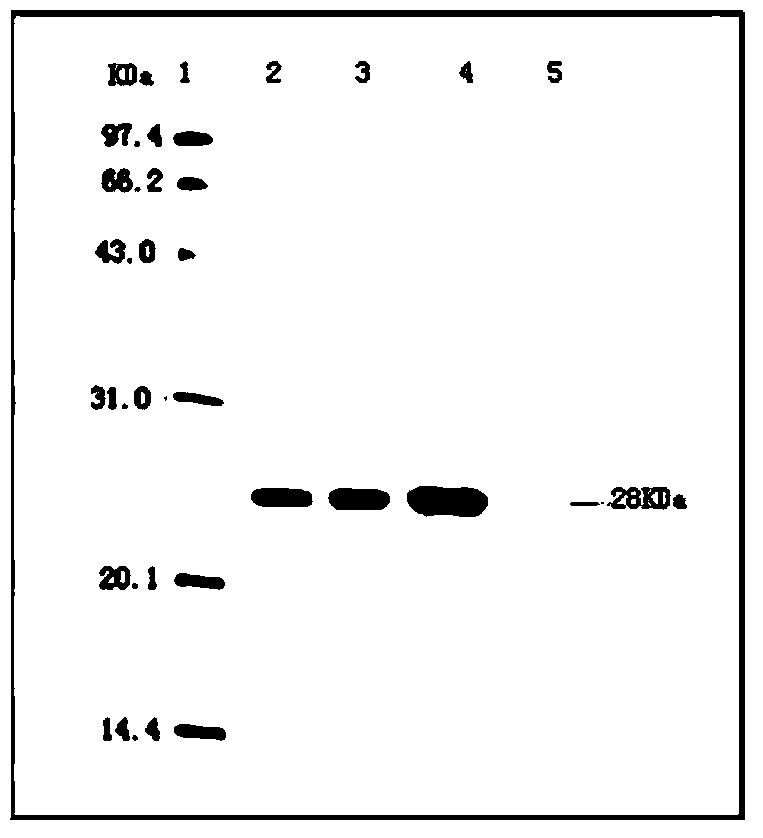
[0067] Embodiment 1: Preparation and inspection of anti-porcine circovirus type 2 and porcine infectious pleuropneumonia infection vaccine composition
[0068] 1. Preparation of bacteria (virus) species for production
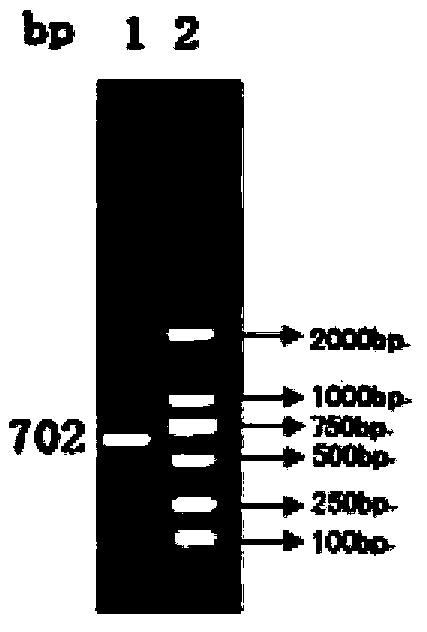
[0069] The preparation of porcine circovirus type 2 SH strain: virus seeds are used MEM medium ( Invitrogen Company) was diluted 10 times, inoculated in PK15 (ATCC, deposit number CCL-33) cell culture according to 5% volume, adsorbed at 37°C for 30min, added D-glucosamine hydrochloride containing 4% calf serum and 2mmol / L MEM cell maintenance medium, cultivated at 37°C for 4 days, freeze-thawed 2-3 times, harvested the virus, and the virus titer was 10 6.5 TCID 50 / ml.
[0070] Preparation of Actinobacillus pleuropneumoniae strains: Streak inoculation of Actinobacillus pleuropneumoniae type 1 LC strain, type 5 YC strain and type 7 YS strain in TSA / NAD plates were cultured at 37°C for 24 hours, and more than 5 typical colonies were selected each. After pas...
Embodiment 2
[0126] Example 2: Comparison of PCV2 inactivated whole virus antigen vaccine to PCV2 challenge immune effect and PCV2 inactivated virus antigen and porcine Actinobacillus pleuropneumoniae inactivated antigen vaccine composition comparison of PCV2 challenge immune effect
[0127] Antigens prepared in Example 1 (i.e. K001 batch of antigens, see Table 2) were used to prepare porcine circovirus type 2 and Actinobacillus pleuropneumoniae (serum type 1+serum type 4+serum type 5) vaccine compositions (V1) respectively and (V2), porcine circovirus vaccine (V3) and (V4), the specific formula is as follows:
[0128] 1. The total volume of porcine circovirus type 2 and Actinobacillus pleuropneumoniae (serotype 1 + serotype 4 + serotype 5) vaccine composition (V1) is about 100ml:
[0129]
[0130] 2. Porcine circovirus type 2, Actinobacillus pleuropneumoniae (serotype 1 + serotype 4 + serotype 5) vaccine composition (V2) has a total volume of about 100ml:
[0131]
[0132] 3. The t...
Embodiment 3
[0151] Example 3: Comparison of the protective effects of porcine infectious pleuropneumonia inactivated antigen vaccine composition, PCV2 inactivated virus antigen, and porcine infectious pleuropneumonia inactivated antigen vaccine composition against Actinobacillus pleuropneumoniae
[0152] Using the antigen K001 batch prepared in Example 1, the vaccine composition (V1), porcine circovirus type 2, Actinobacillus pleuropneumoniae (serum type 1 + serum type 5 + serum type 7) vaccine composition (V1), Actinobacillus pleuropneumoniae inactivated vaccine (serotype 1 + serotype 5 + serotype 7) (V2), the specific formula is as follows.
[0153] (1) Porcine circovirus type 2, Actinobacillus pleuropneumoniae (serotype 1 + serotype 5 + serotype 7) vaccine composition (V1):
[0154]
[0155] (2) Actinobacillus pleuropneumoniae inactivated vaccine (serotype 1 + serotype 5 + serotype 7) inactivated vaccine (V2):
[0156]
[0157] Select 50 weaned piglets aged 35 to 40 days and div...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com