Application of truncated fragment of porcine nlrp3 as antigenic structural protein
A protein and monoclonal antibody technology, applied in anti-animal/human immunoglobulin, recombinant DNA technology, introduction of foreign genetic material using vectors, etc., can solve the problems that do not exist, and achieve stable performance and high antigen titer. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Embodiment 1, antigen preparation
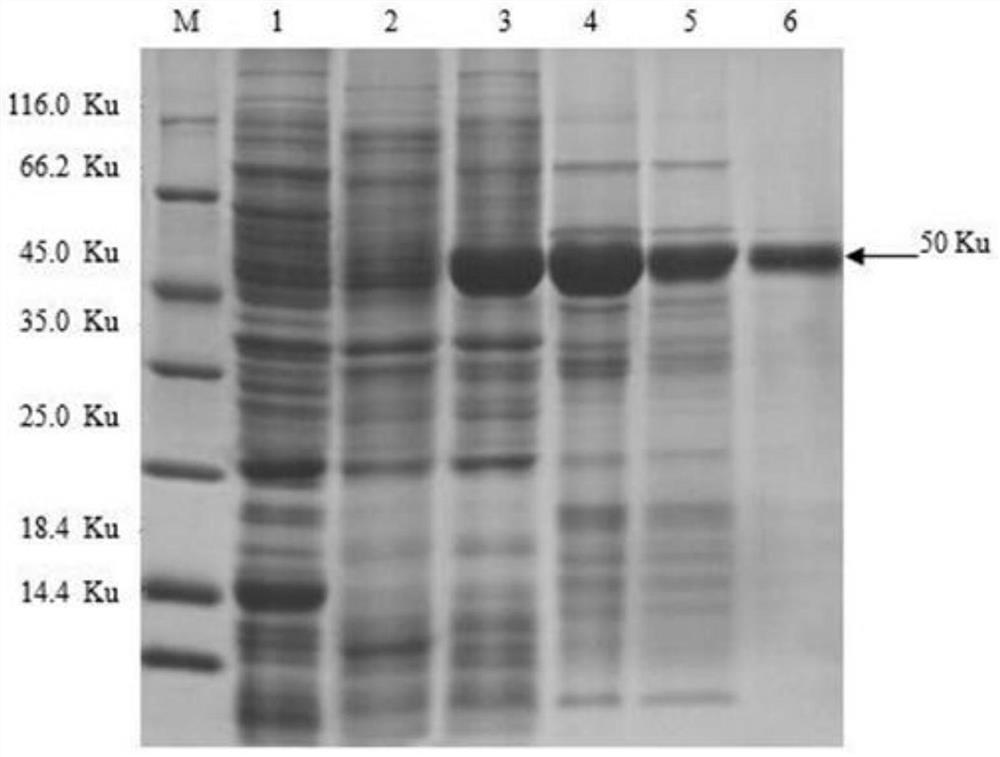
[0027] In order to study new therapeutic targets for porcine inflammatory diseases, correctly treat, prevent and control inflammatory diseases caused by viruses and bacteria, etc., it is planned to prepare porcine NLRP3 monoclonal antibody. First, a truncated porcine NLRP3 fragment consisting of 288 amino acid residues is selected. The molecular weight is about 50KDa. As the NLRP3 antigen structural protein, the amino acid sequence of its NLRP3 truncated fragment is shown in SEQ ID NO.1, and the nucleotide sequence encoding the amino acid is shown in SEQ ID NO.2.
[0028] The nucleotide sequence shown in SEQ ID NO.2 was connected into the expression vector pET-32a through BamH I and Xho I to obtain the recombinant expression vector pET32a-NLRP3. After sequencing and verification, the expression vector with the correct target gene sequence was selected for protein expression. Express.
[0029] The specific expression method is as foll...
Embodiment 2
[0031] Embodiment 2, animal immunization
[0032] Experimental animals and immunization methods: select 5 Balb / C mice aged 5-8 weeks, use NLRP3 recombinant protein prepared in Example 1 as antigen, inject 100 μg antigen / experimental mouse at multiple points on the back, and inject 50 μg antigen for booster immunization For experimental mice, Freund's complete adjuvant was mixed with an equal volume of antigen for the first injection, and Freund's incomplete adjuvant was mixed with an equal volume of antigen for booster injection. The specific immunization time and cycle are shown in Table 1.
[0033] Table 1. Immunization cycle
[0034] Experiment process date primary exemption 2018.07.15 first booster 2018.07.23 second booster 2018.07.31 third booster 2018.08.08 fusion 2018.08.16 filter 2018.08.24 sub-screening 2018.09.01 Secondary screening 2018.09.09 ascites 2018.09.24
[0035] Antiserum testing:
[003...
Embodiment 3
[0043] Embodiment 3, cell fusion and subcloning
[0044] (1) Preparation of myeloma cells: One week before fusion, SP2 / 0 cells were revived and cultured to logarithmic phase normally.
[0045] (2) Spleen cell preparation: the mice to be fused were selected, sacrificed by cervical dislocation on the day of fusion, the spleen was taken, and the splenocytes were collected and counted according to the standard procedure.
[0046] (3) Cell fusion: mix myeloma cells and spleen cells at a ratio of 1:3-1:10, perform cell fusion operation according to the standard procedure, and then culture with HAT DMEM complete medium. Hybridomas can be seen 3 days after fusion Cells were replaced with 1 / 2HAT complete medium on the 7th day, and 1 / 2HT medium on the 8th day, and the screening test was started about 10 days after fusion.
[0047] Results of cell fusion: after fusion, cultured with HAT selective medium, observed under a microscope, many growing hybridoma cells were seen, proving that t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com