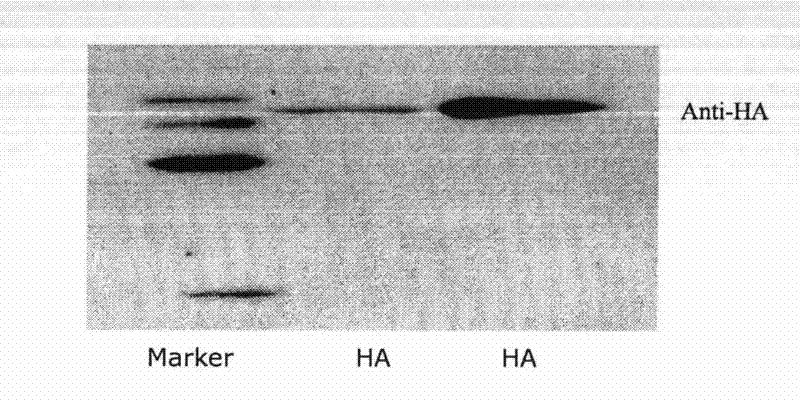
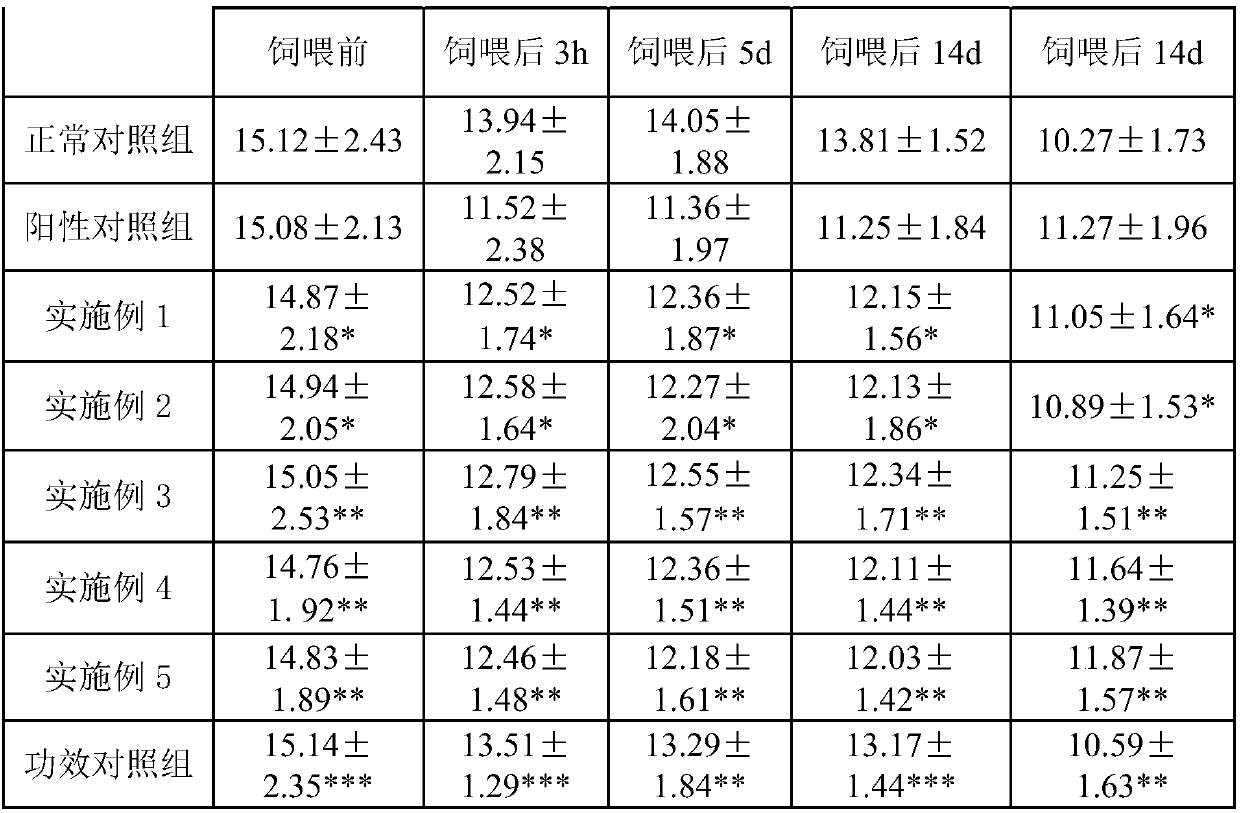
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
183 results about "Hematinic" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A hematinic is a nutrient required for the formation of blood cells in the process of hematopoiesis. The main hematinics are iron, B12, and folate. Deficiency in hematinics can lead to anaemia. In cases of hematinic deficiency, hematinics can be administered as medicines, in order to increase the hemoglobin content of the blood.
Transdermal method and apparatus
Owner:ALDRED KATHERINE M
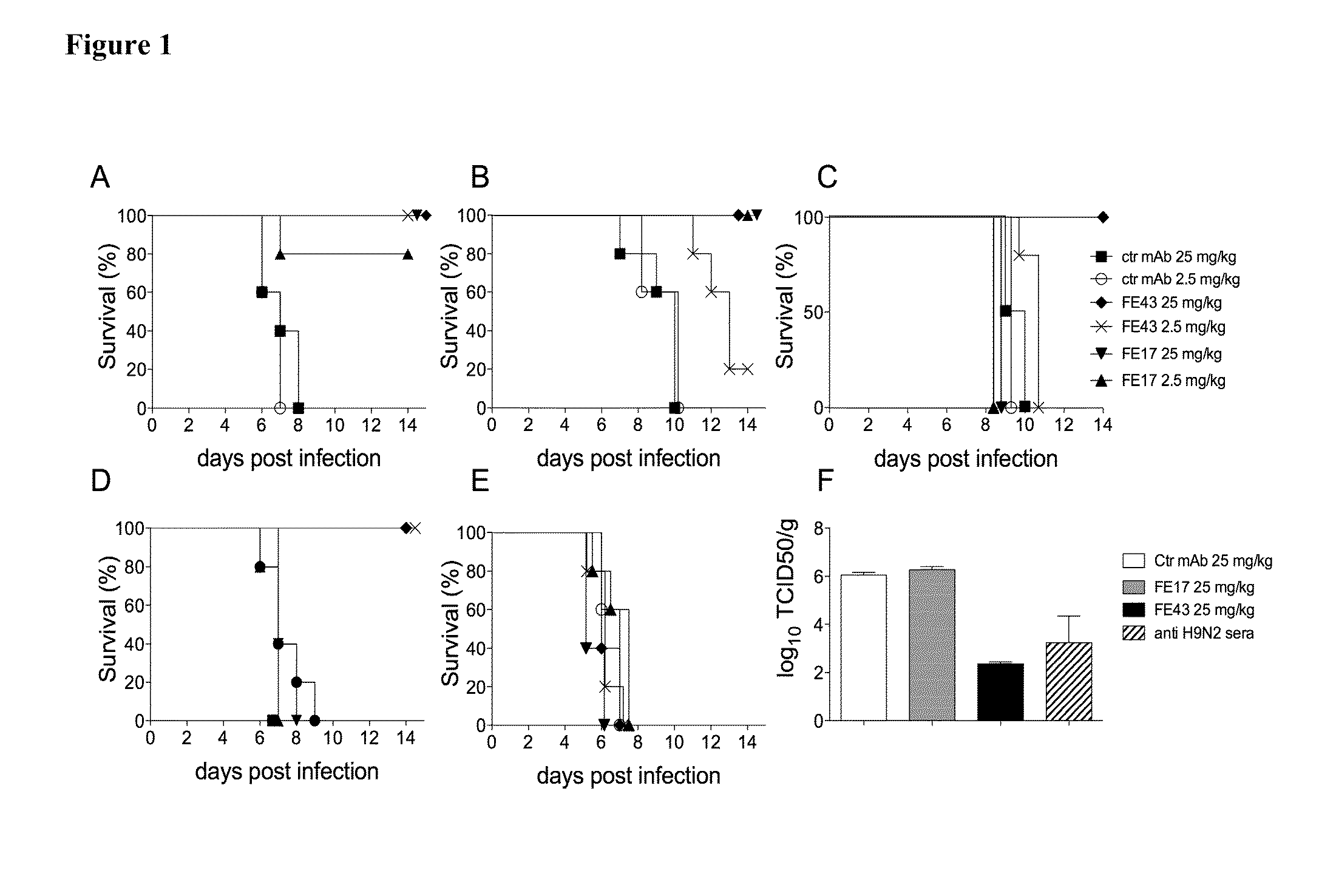
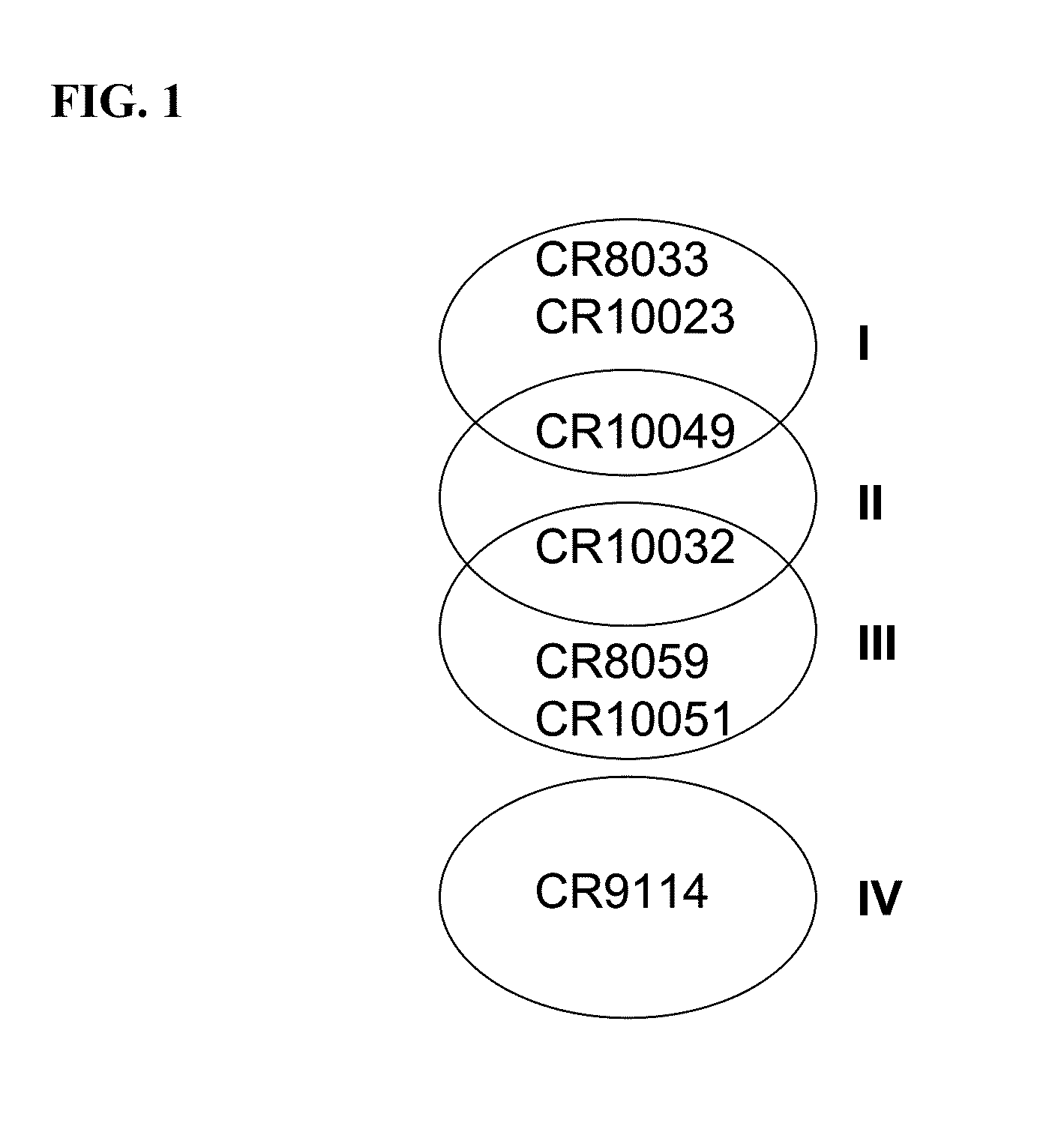
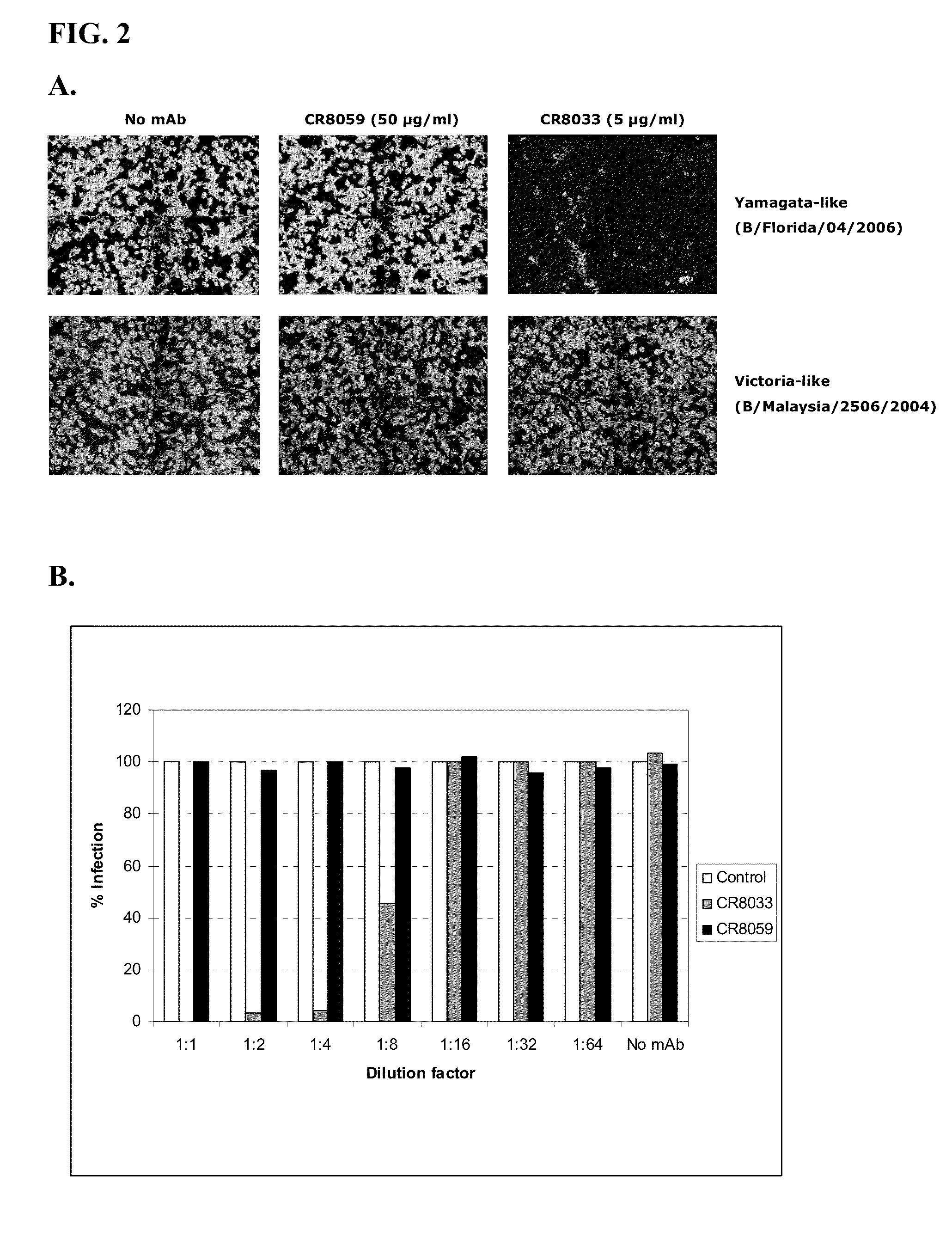
Monoclonal antibodies binding to avian influenza virus subtype h5 haemagglutinin and use thereof
InactiveUS20090068637A1Bioreactor/fermenter combinationsBiological substance pretreatmentsHemagglutininAvian influenza virus
The present application provides monoclonal antibodies that specifically bind to the hemagglutinin of avian influenza virus subtype H5, as well as monoclonal antibodies capable of blocking at least 50% of the hemagglutinin binding activity of these monoclonal antibodies. Such antibodies are useful, for example, in the detection, diagnosis, prevention, and treatment of avian influenza virus. Also provided herein are hybridoma cell lines, isolated nucleic acid molecules, and short peptides related to the monoclonal antibodies provided herein, and pharmaceutical compositions and kits containing the monoclonal antibodies provided herein.
Owner:HX DIAGNOSTICS INC
Anti-(influenza a virus subtype h5 hemagglutinin) monoclonal antibody
InactiveUS20110065095A1Prevention of prevalenceFaster assayMicrobiological testing/measurementBiological material analysisHemagglutininEpitope
A method of immunoassay of H5 subtype influenza A virus by which the virus can be accurately assayed even in cases where a certain level of mutation has occurred in the H5 subtype influenza A virus, and a kit therefor, and a novel anti-H5 subtype influenza A virus monoclonal antibody which can be used for the immunoassay are disclosed. The antibody or an antigen-binding fragment thereof of the present invention undergoes antigen-antibody reaction with hemagglutinin of H5 subtype influenza A virus, and the corresponding epitope of the antibody or an antigen-binding fragment thereof is located in a region other than the receptor subdomain (excluding C-terminal region thereof consisting of 11 amino acids), which antibody or an antigen-binding fragment thereof does not have neutralizing activity against the influenza A virus.
Owner:FUJIREBIO CO LTD +1
Neutralizing Anti-influenza a virus antibodies and uses thereof
The invention relates to antibodies and antigen binding fragments thereof, that bind to hemagglutinin and neutralize infection of at least two different group 1 subtypes or at least two different group 2 subtypes of influenza A virus. The invention also relates to nucleic acids that encode, immortalized B cells and cultured single plasma cells that produce, and to epitopes that bind to, such antibodies and antibody fragments. In addition, the invention relates to the use of the antibodies, antibody fragments, and epitopes in screening methods as well as in the diagnosis, treatment and prevention of influenza A virus infection.
Owner:INSTITUTE FOR RESEARCH IN BIOMEDECINE
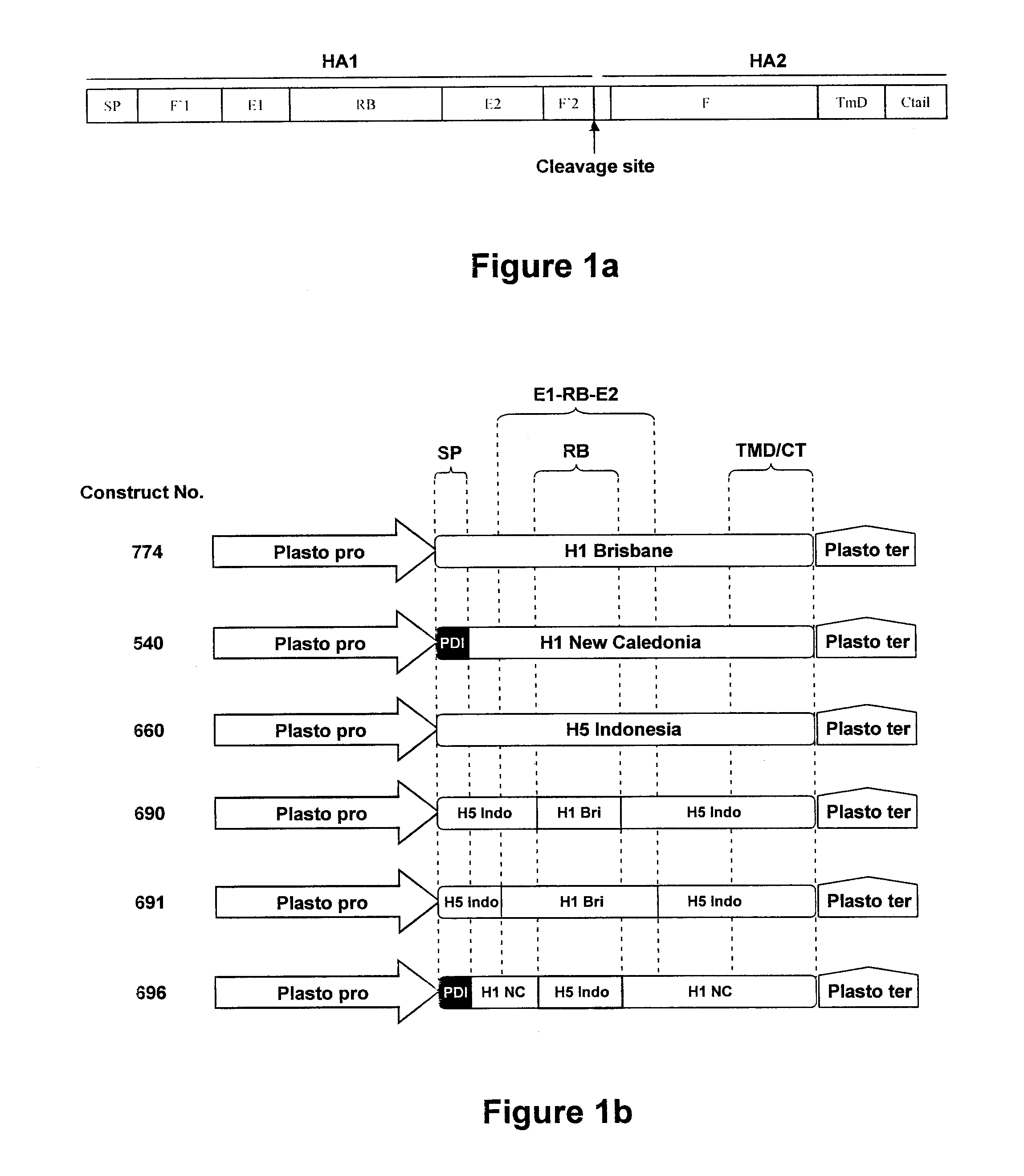
Chimeric Influenza Virus-Like Particles Comprising Hemagglutinin
ActiveUS20120189658A1Easy to captureStrong immune responseSsRNA viruses negative-senseAntibody mimetics/scaffoldsHemagglutininVirus-like particle
A method for synthesizing chimeric influenza virus-like particles (VLPs) within a plant or a portion of a plant is provided. The method involves expression of chimeric influenza HA in a plant or a portion of a plant. The invention is also directed towards a VLP comprising chimeric influenza HA protein and plants lipids. The invention is also directed to a nucleic acid encoding chimeric influenza HA as well as vectors. The VLPs may be used to formulate influenza vaccines, or may be used to enrich existing vaccines.
Owner:MEDICAGO INC
Recombinant multivalent viral vaccine
InactiveUS7087234B1Elicit immune responseReadily apparentSsRNA viruses negative-senseSsRNA viruses positive-senseHemagglutininViral Vaccine
The present invention relates to multivalent recombinant raccoon poxviruses, containing more than one exogenous gene inserted into either the thymidine kinase gene, the hemagglutinin gene, or a combination thereof. Disclosed is the use of the multivalent recombinant raccoon poxviruses as vaccines to immunize felines against subsequent challenge by feline pathogens. Also disclosed is a method of making a multivalent recombinant raccoon poxvirus by a recombination process involving the construction of an insertion vector into which the exogenous genes are inserted, and flanking the inserted genes are sequences which can recombine into the raccoon poxvirus thymidine kinase gene, or the hemagglutinin gene, or a combination thereof; introducing both the insertion vector containing the exogenous genes, and raccoon poxvirus into susceptible host cells; and selecting the recombinant raccoon poxvirus from the resultant plaques.
Owner:CORNELL RES FOUNDATION INC +1
Influenza b viruses having alterations in the hemaglutinin polypeptide
InactiveUS20100322969A1SsRNA viruses negative-senseViral antigen ingredientsInfluenza B virusesHematinic
The present invention encompasses methods of producing influenza B viruses in cell culture. The influenza B viruses may have desirable characteristics, such as enhanced replication in eggs and may be used, for example, in vaccines and in methods of treatment to protect against influenza B virus infection.
Owner:MEDIMMUNE LLC
Human binding molecules capable of neutralizing influenza a viruses of phylogenetic group 1 and phylogenetic group 2 and influenza b viruses
ActiveUS20140120113A1High economic impactContinuing riskSugar derivativesGenetic material ingredientsHemagglutininEpitope
The present disclosure relates to binding molecules, such as human monoclonal antibodies, that bind to an epitope in the stem region of hemagglutinin of influenza A viruses of phylogenetic group 1 and group 2, as well as influenza B viruses, and have a broad neutralizing activity against such influenza viruses. The disclosure provides nucleic acid molecules encoding the binding molecules, their sequences and compositions comprising the binding molecules. The binding molecules can be used in the diagnosis, prophylaxis and / or treatment of influenza A viruses of phylogenetic groups 1 and 2, as well as influenza B viruses.
Owner:JANSSEN VACCINES & PREVENTION BV
Polypeptide analogue for blocking NR2B signal path and preparation method and medical usage thereof
InactiveCN101134780AAvoid space obstructionNervous disorderPeptide/protein ingredientsNR1 NMDA receptorDisease
The present invention provides one kind of fusion protein in C-terminal of recombinant NMDA receptor NR2B subtype and produced through gene engineering process, polynucleotides encoding the fusion protein, and process of preparing and purifying the fusion protein. The fusion protein consists of the PSD95 linking region segment of NMDA receptor NR2B subtype and the transduction region segment of HIV coded Tat protein, and has one hemagglutinin marker segment for influenza virus inserted between the two above said segments. The fusion protein may be expressed effectively in colibacillus, has simple purifying process and is suitable for production in large scale. The Tat-HA-NR2B9C polypeptide of the present invention can block the interaction between NMDA receptor and PSD95, inhibit NMDA receptor mediated excitable neurotoxicity effect and protect nerves. It may be applied in treating cerebral apoplexy and relevant nerve diseases.
Owner:NANJING MEDICAL UNIV
Peptide vaccine for influenza virus
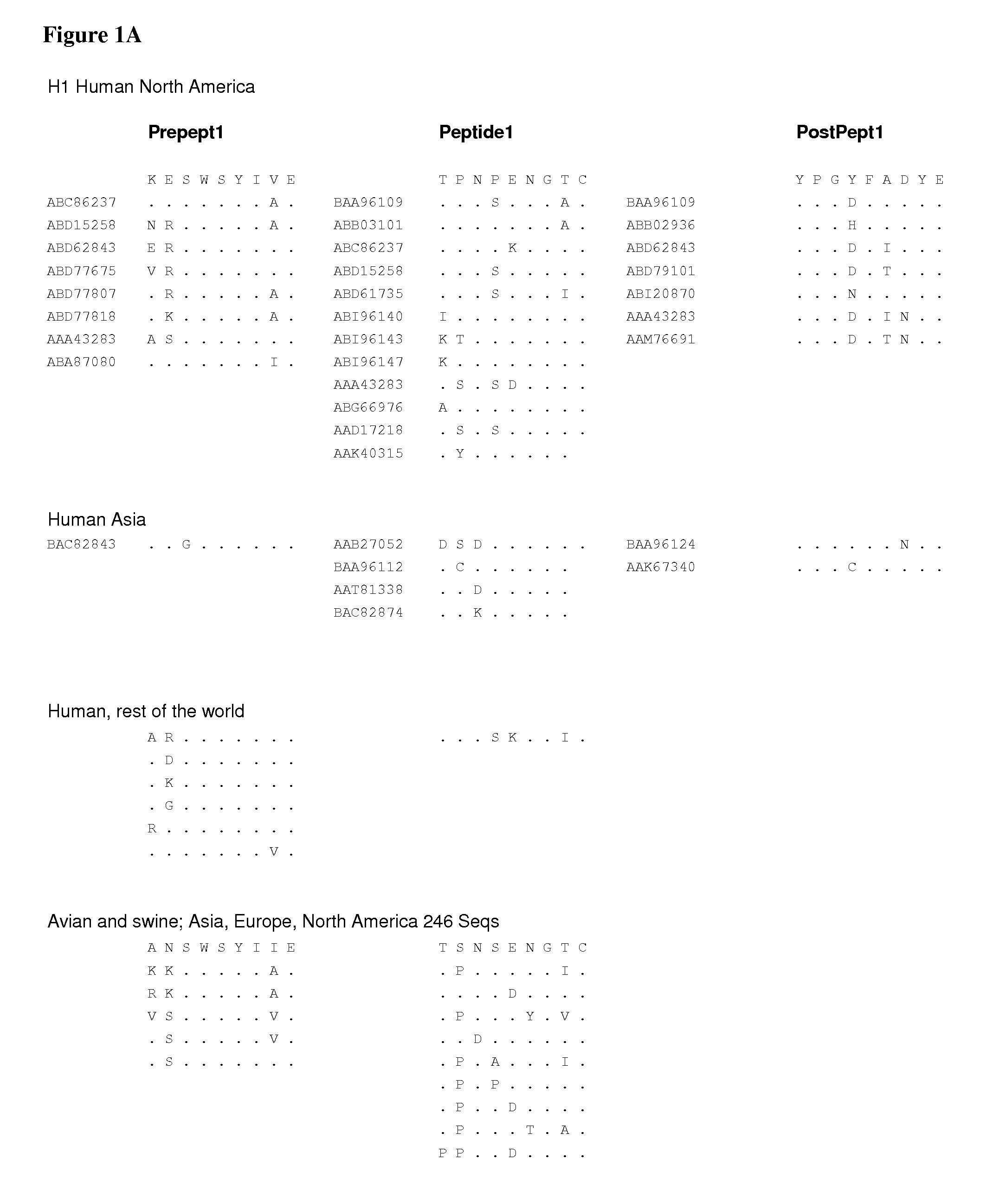
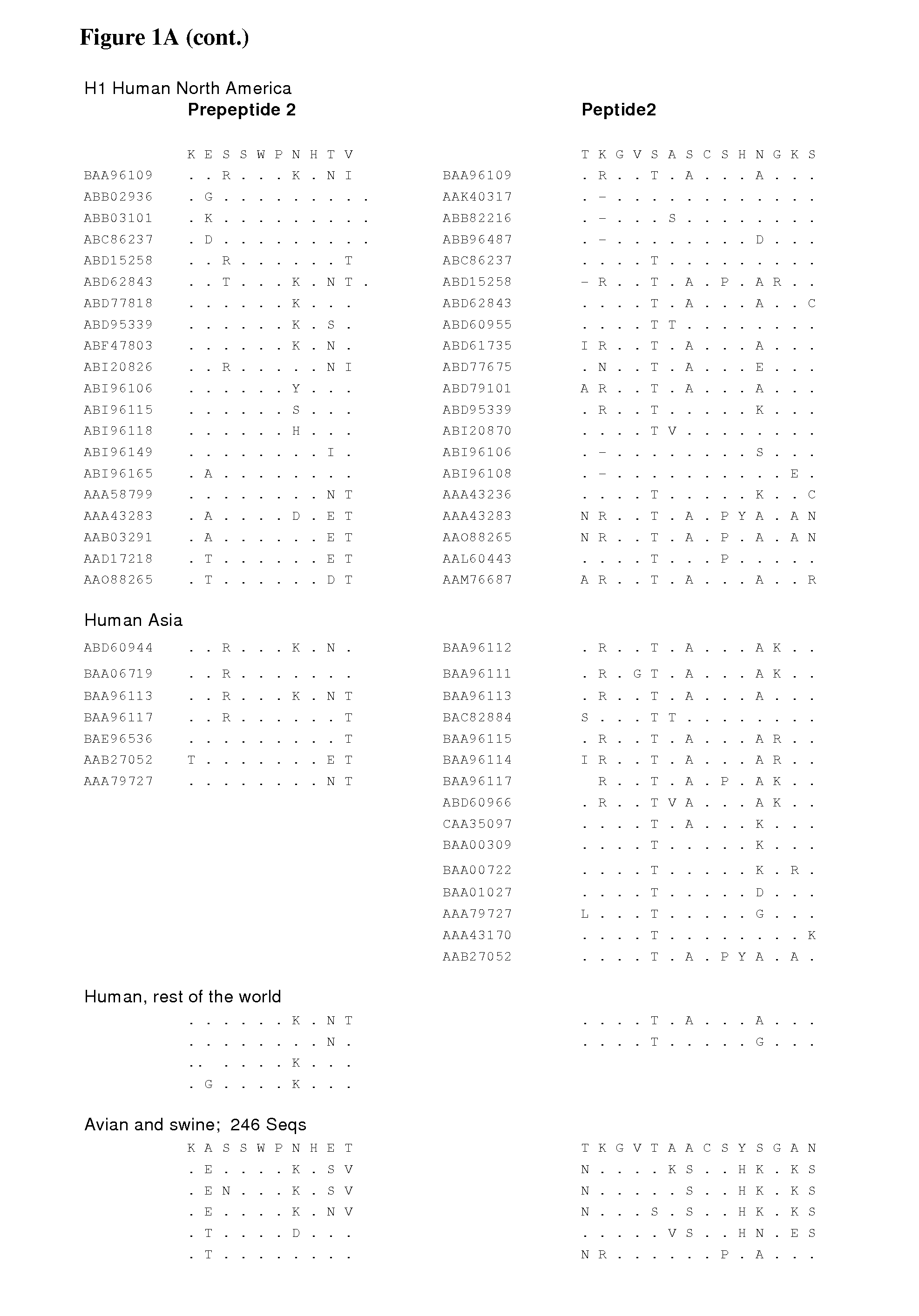
The invention provides peptide epitopes for use in the prevention and / or treatment of influenza or for the development of such treatment or vaccine against influenza. The invention also relates to a method for evaluating the potential of a chemical entity, such as an antibody, to bind to a peptide epitope derived from the divalent sialoside binding site of hemagglutinin protein of influenza virus, and to conjugates containing one or more such peptide epitopes. The peptide epitopes of the invention are cyclic peptides comprising a 7-mer peptide derived from H1, H3 or H5 hemagglutinin of influenza virus. The 7-mer peptide has a sequence corresponding to the loop sequence at positions 220-226 of X31-hemagglutinin.
Owner:GLYKOS FINLAND
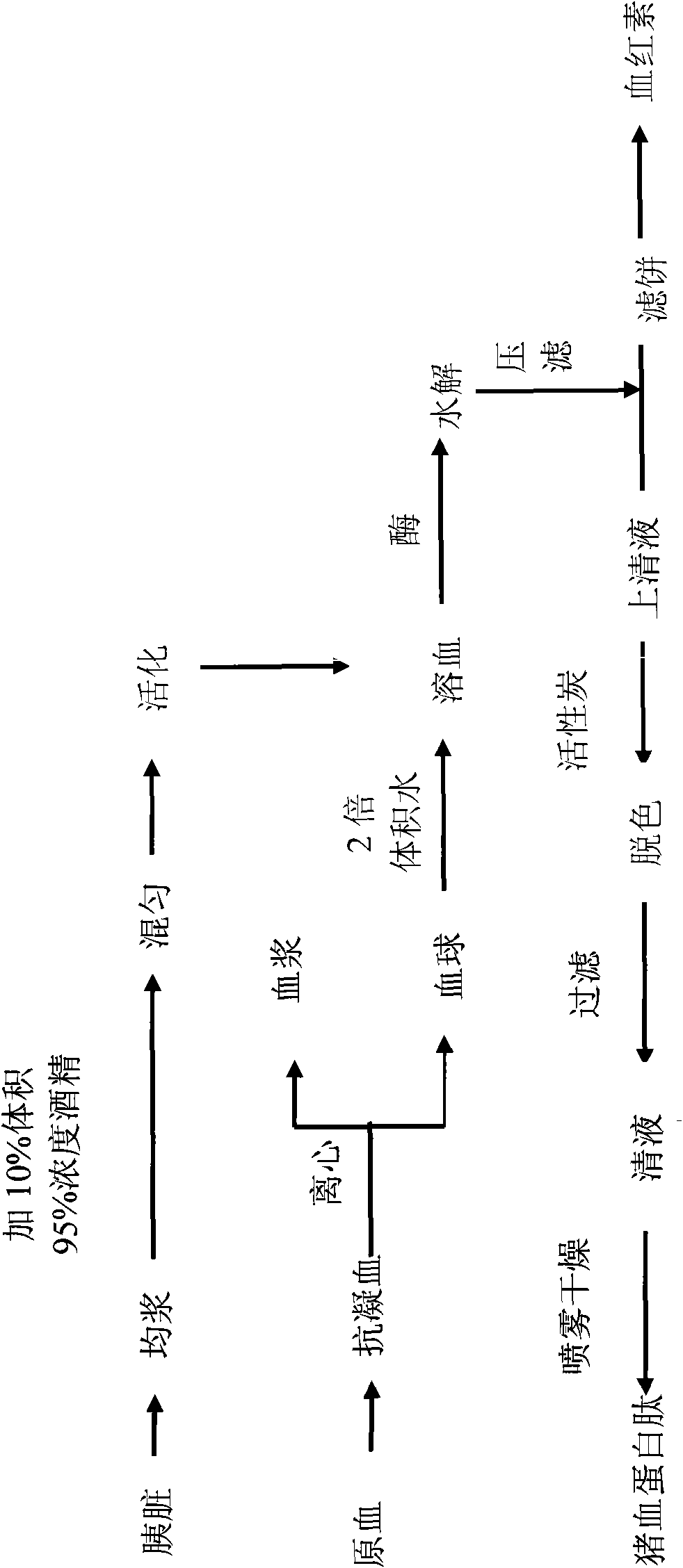
Technology using swine fresh pancreatin to produce swine blood protein peptide and haemoglobin
The invention relates to a method using pancreatin-hydrolyzed swine blood corpuscle protein activated by swine fresh pancreas to produce swine blood protein peptide and haemoglobin, belonging to the field of feed additive. The invention takes fresh healthy swine blood corpuscle liquid as a raw material, pancreatin activated by swine fresh pancreas is hydrolyzed and filtered, the filtered supernatant is decolorized, then, the obtained protein liquid is dried to obtain the golden swine blood protein peptide, and the filter cake is dried to obtain the haemoglobin. The technical method has the following innovation points: 1, swine pancreas is taken as the raw material which is fresh and available, and the quality is controllable; 2, pancreatin activation method is simple, enzyme systems are rich, the activated enzyme has high activity, and the hydrolysis effect is good; 3, the swine fresh pancreatin has low cost, which is easy to carry out expanded production; 4, the swine blood corpuscle protein is hydrolyzed by pancreatin which can efficiently separate the haemoglobin thereof and can further prepare haemoglobin by drying the filter cake as the hydrolysate product, thus greatly improving the rate of multipurpose utilization of the swine blood corpuscle protein and avoiding resource waste.
Owner:天津宝迪农业科技股份有限公司
Chimeric influenza virus-like particles
InactiveUS20100074915A1Improving immunogenicitySufficient quantitySsRNA viruses negative-senseHydrolasesHemagglutininVirus-like particle
Chimeric Influenza virus-like particles including gag polypeptides are described. Virus-like particles are generated with a gag polypeptide, a neuraminidase polypeptide and optionally a hemagglutinin polypeptide. Preferred methods of generation include expression in insect cells.
Owner:TAKEDA VACCINES INC
Six-flavor hematinic capsule, its quality control method and application thereof
ActiveCN103381217ARealize authenticationAccurate measurementSenses disorderComponent separationBlurred visionLithospermum
The invention discloses a six-flavor hematinic capsule, its quality control method and an application thereof. The six-flavor hematinic capsule is prepared by adding auxiliary materials into raw materials consisting of Chinese angelica, Ligusticum wallichii, Radix Astragali, prepared rehmannia root, lithospermum and white peony root. According to the quality control method of the six-flavor hematinic preparation, whether the six-flavor hematinic capsule contains white peony root, Chinese angelica, Radix Astragali, prepared rehmannia root, lithospermum, Ligusticum wallichii and components is identified by thin layer chromatography; by high-performance liquid chromatography, in vitro dissolution behavior of the six-flavor hematinic capsule is determined, and effective component groups are identified by fingerprint; verbascoside and calycosin glucoside are used as reference substances to simultaneously determine contents in the six-flavor hematinic capsule; and the content of a volatile component ligustilide in the six-flavor hematinic capsule is determined by a gas chromatographic method. The method provided by the invention is simple to operate, is accurate and advanced, has good linear relation, reappearance, precision, stability and recovery rate, can be adopted to effectively control product quality and guarantee curative effect of the product. The invention also provides an application in the preparation of a medicine for treating eye damage and blurred vision.
Owner:GUANGDONG GUOYUAN GUOYAO PHARMA CO LTD
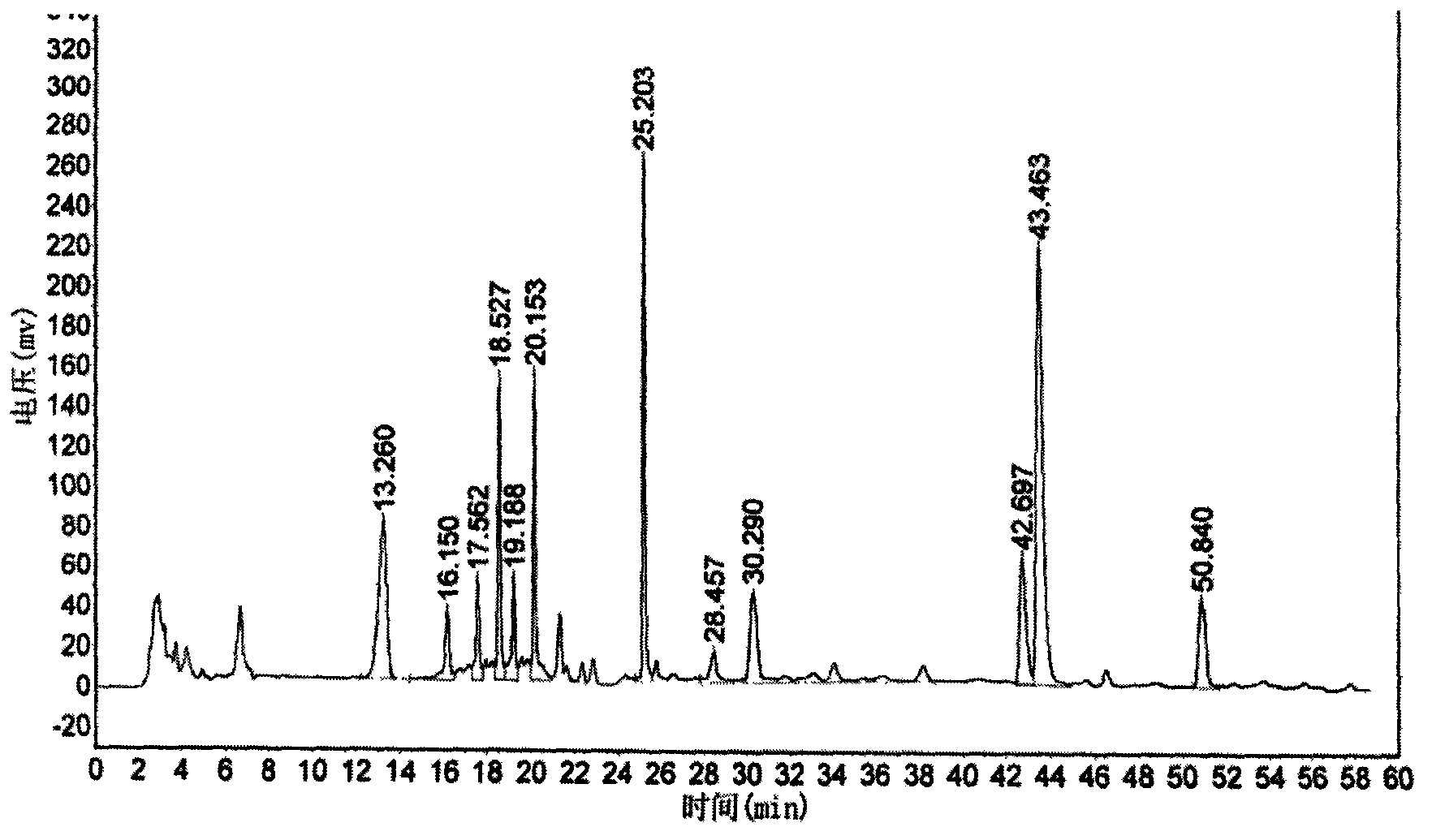
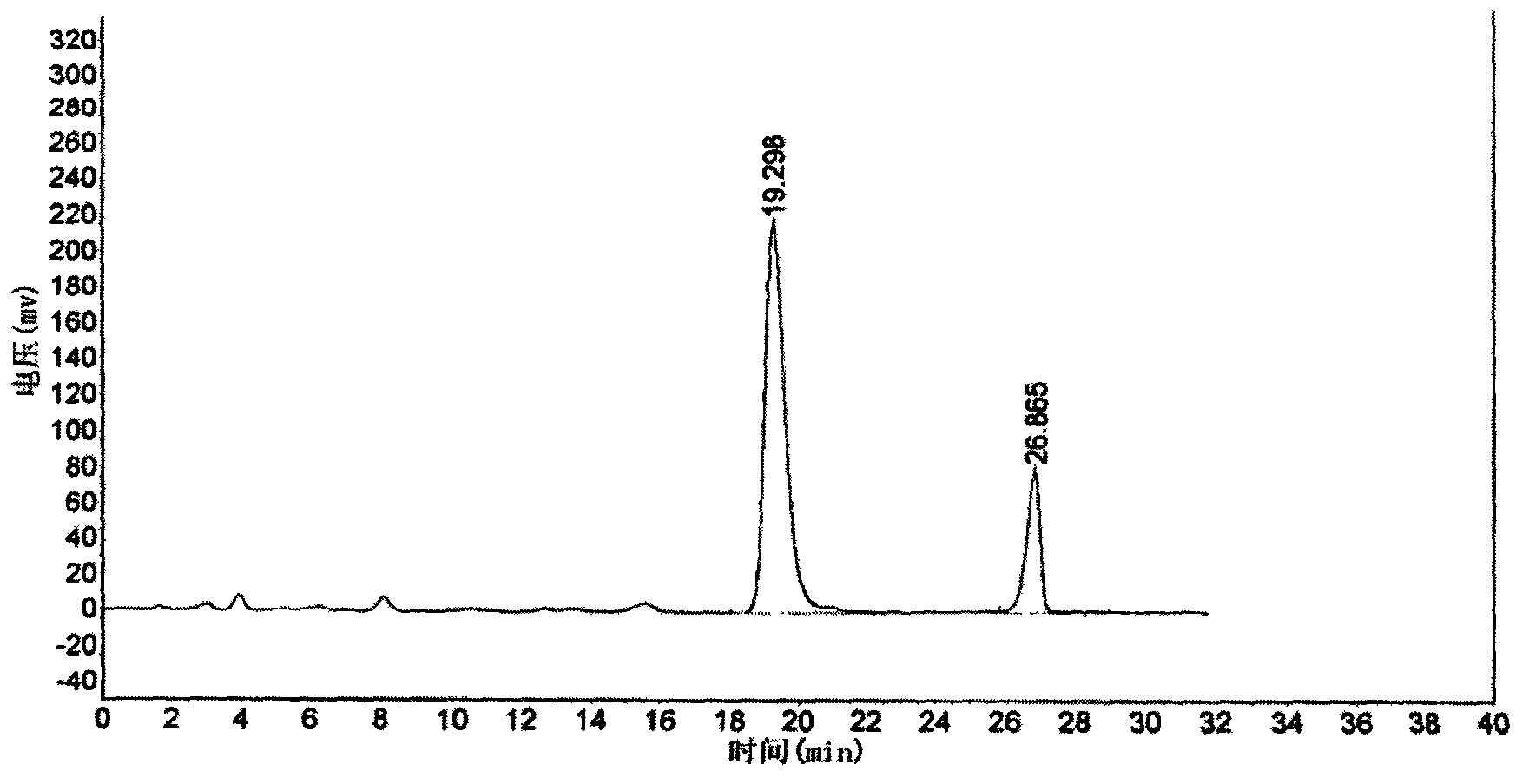
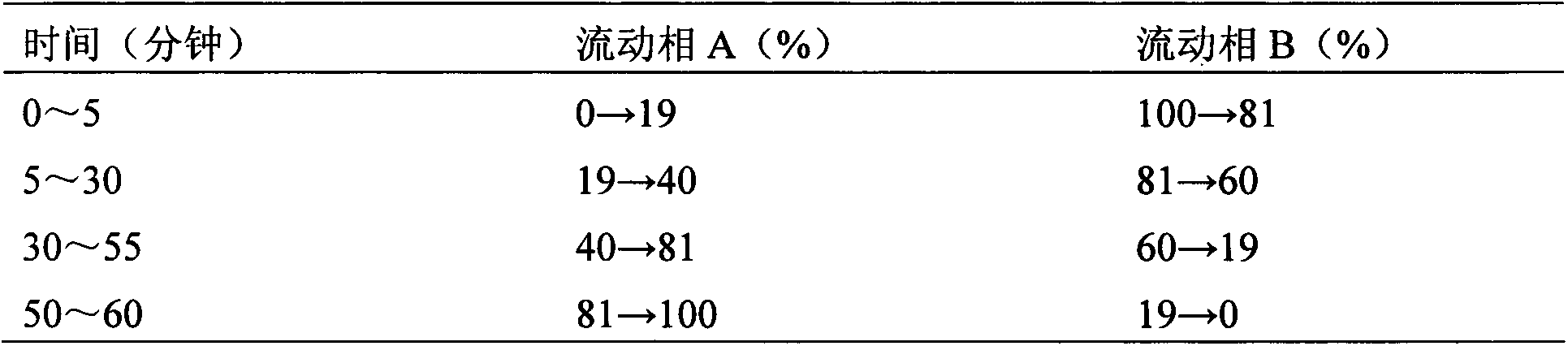
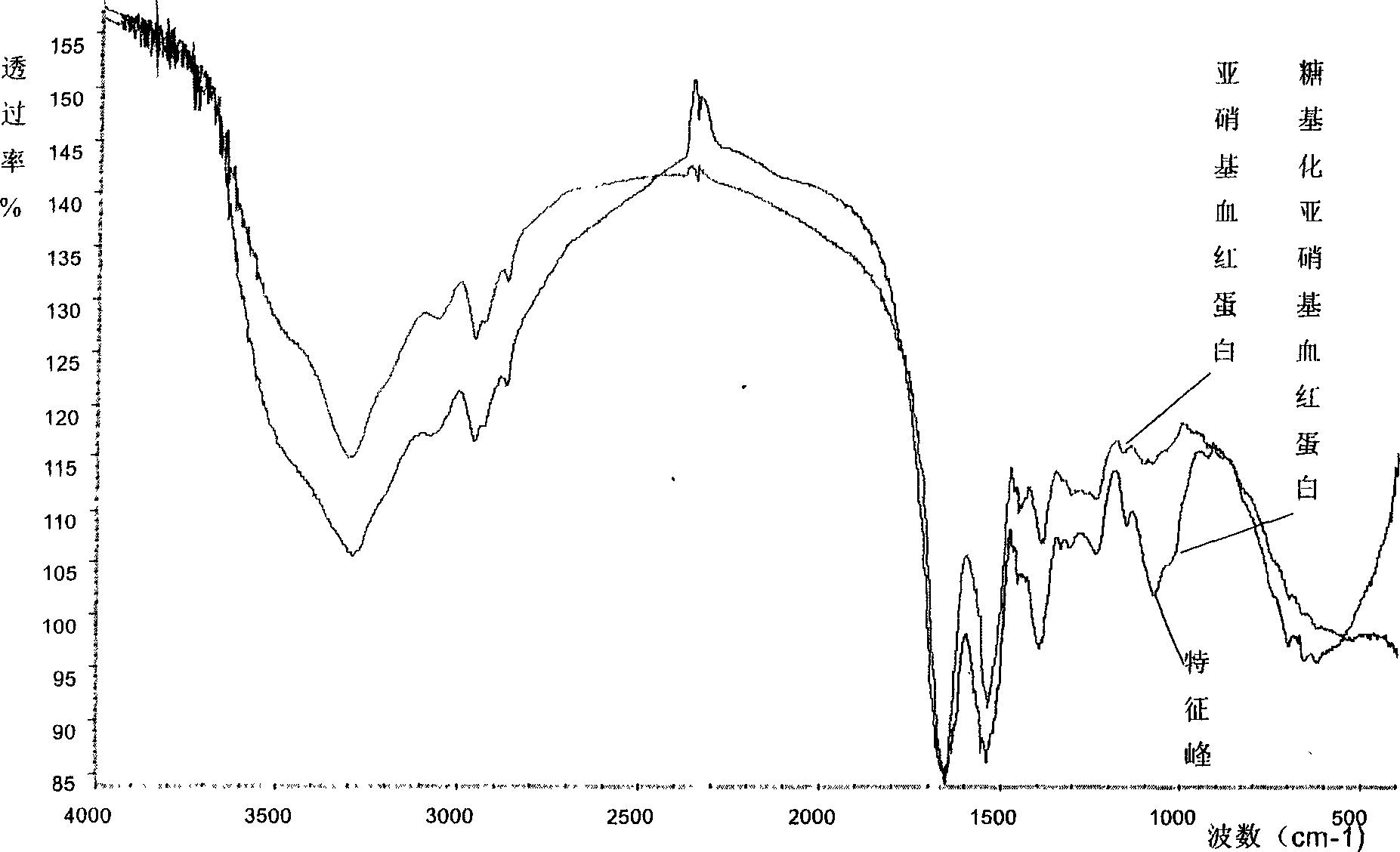
Glyconitroso ferrohemoglobin pigment, it preparation and application
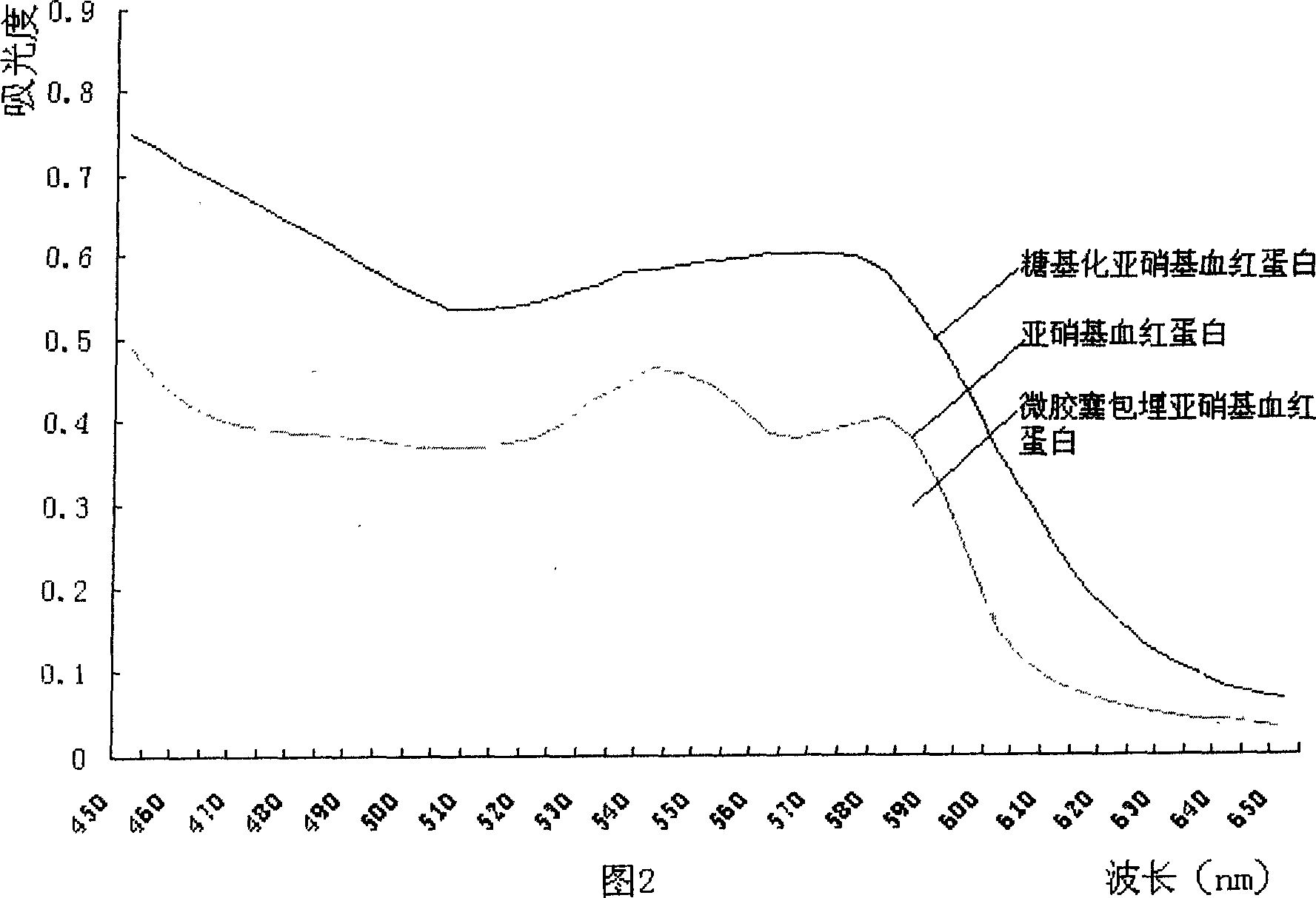
InactiveCN1650748AImprove thermal stabilityGood storage stabilityFood preparationDispersityFood additive
A glyosylated nitroso hemoglobin pigment for food is prepared from the hemoglobin in the blood of animals or fowls through reacting on sodium nitrite or NO to obtain nitroso hemoglobin, reacting on saccharide to obtain glyosylated nitroso hemoglobin pigment and spray drying. It can be mixed with antioxidizing agent and antiaseptic to obtain a preserving system used for meat. Its advantages are high stability and dispersity, and high effect to decrease the residue of sodium nitrate in preserved meat.
Owner:上海华宝孔雀香精有限公司
Influenza vaccines with reduced amount of emulsion adjuvant
InactiveUS20090304742A1Eliminate needAvoid the needSsRNA viruses negative-senseViral antigen ingredientsHemagglutininInfluenza vaccine
Influenza vaccines with oil-in-water emulsion adjuvants are known. The amount of emulsion adjuvant required for an influenza vaccine can be reduced, thereby allowing more vaccines to be made from a given amount of emulsion, and / or minimizing the amount of emulsion that has to be produced for a given number of vaccine doses. These vaccines can conveniently be made by mixing (i) an oil-in-water emulsion and (ii) an aqueous preparation of an influenza virus antigen. In one aspect, substantially equal volumes of components (i) and (ii) are used; in another aspect, an excess volume of component (ii) is used. When using substantially equal volumes, component (ii) has a hemagglutinin concentration of more than 60 μg influenza virus strain per ml. Components (i) and (ii) can be presented in kit form.
Owner:NOVARTIS AG
Human binding molecules capable of binding to and neutralizing influenza b viruses and uses thereof
ActiveUS20140065165A1Microbiological testing/measurementBiological material analysisHemagglutininMonoclonal antibody
Described are binding molecules, such as human monoclonal antibodies, that bind to hemagglutinin of influenza B viruses, and have a broad neutralizing activity against such influenza viruses. These binding molecules do not bind to hemagglutinin of influenza A viruses. Further provided are nucleic acid molecules encoding the binding molecules, and compositions comprising the binding molecules. The binding molecules can be used in the diagnosis of, prophylaxis against, and / or treatment of influenza B virus infections.
Owner:JANSSEN VACCINES & PREVENTION BV
Iron supplementary liposome iron and method of preparing the same
InactiveCN101254207AHigh encapsulation efficiencyReduce dosageHeavy metal active ingredientsOrganic active ingredientsSolubilityCholesterol
The invention relates to a liposomal iron prepared as a novel iron-supplementing agent by a rotary film ultrasonic method. The liposomal iron is prepared from heme iron and / or inorganic iron, cholesterol and lecithin at a weight ratio of (0.1-200):(1-10):(10-100) by dispersing lecithin, and cholesterol ethanol solution into iron solution containing PBS under a specific condition. The inventive liposomal iron iron-supplementing agent has no toxicity or immunogenicity, has targeting ability, and can be used as pharmaceutical carrier. Clinically, the liposomal iron iron-supplementing agent can reduce drug dose, improve absorption efficiency, reduce toxicity, remarkably improve the solubility and stability of heme iron, and improve bioavailability. The rotary film ultrasonic method can obtain liposome with high encapsulation efficiency, can effectively remove organic (ethanol) residues, and has the advantages of easily controlled process conditions and simple operation.
Owner:HEBEI NORMAL UNIV
Structure and application of influenza virus hemagglutinin protein binding polypeptide
InactiveCN102268072ACytopathic inhibitionNo obvious toxicityPeptide/protein ingredientsPeptidesDiseaseHemagglutinin
Belonging to the technical field of biomedicine, the invention relates to a sequence and structure of a polypeptide able to specifically bind with influenza virus hemagglutinin protein, and application of the polypeptide in anti-influenza viruses. By expressing purified influenza virus hemagglutinin protein and screening a random peptide library with a phage display technology, a polypeptide specifically bound with influenza virus hemagglutinin and equipped with sequences numbered 1-18 can be obtained. As a hemagglutinin-binding peptide can hinder the combination of hemagglutinin and a host cell receptor, so the influenza virus can be inhibited from infecting the host cell. Thus, the invention also conducts an anti-influenza virus activity study to the hemagglutinin-binding peptide selected from the phase peptide library, and finds that a polypeptide H17, with a sequence of NH2-SHGRITFAYFAN-COOH, can effectively inhibit the influenza virus from infecting the host cell and is of small toxicity. Therefore, the hemagglutinin-binding peptide of the invention and the H17 polypeptide therein with an anti-influenza virus activity are expected to become novel treatment medicaments for treating diseases caused by influenza virus infection and reducing the hazards of diseases caused by influenza viruses.
Owner:INST OF RADIATION MEDICINE ACAD OF MILITARY MEDICAL SCI OF THE PLA
Comprehensive nutrition powder for reducing fat, lowering blood sugar, lowering blood pressure and lowering blood fat, and preparation method thereof
PendingCN110771825ARetain bioactive ingredientsImprove immunityClimate change adaptationFermentationBiotechnologyAnimal science
The invention discloses a comprehensive nutrition powder for reducing fat, lowering blood sugar, lowering blood pressure and lowering blood fat, and a preparation method thereof. The comprehensive nutrition powder comprises nutrients most easily deficient in modern people, auxiliary nutrients for promoting absorption of the nutrients, and effective components for reducing fat, lowering blood sugar, lowering blood pressure and lowering blood fat, and specifically comprises: broccoli powder or carrot powder, black fungus powder, pleurotus eryngii powder, oyster powder, vitamin C, vitamin B2, heme iron, hericium erinaceus cell wall-broken ultrafine powder, composite fermented powder of Chenopodium quinoa and chickpea, olive kernel peptide powder, and composite fermented powder of Sacha Inchikernel and linseed, wherein the pleurotus eryngii powder is dried in the sun. The comprehensive nutrition powder comprehensively considers interaction between various nutrients, eliminates interference of phytic acid on absorption of minerals, and can achieve effects of reducing fat, lowering blood sugar, lowering blood pressure and lowering blood fat from multiple sources and in various aspects.The preparation method can well retain nutrition of dietary ingredients in the comprehensive nutrition powder, and maximize functions of the effective ingredients in reducing fat, lowering blood sugar, lowering blood pressure and lowering blood fat.
Owner:重庆一家人生物科技有限公司
Influenza virus vaccines and uses thereof
ActiveUS20160136262A1SsRNA viruses negative-senseAntibody mimetics/scaffoldsHemagglutininInfluenza virus vaccine
Provided are influenza hemagglutinin stem domain polypeptides comprising (a) an influenza hemagglutinin HA1 domain that comprises an HA1 N-terminal stem segment comprising the amino acids from position 1 to position x, preferably from position p to position x, of the HA1 domain, covalently linked by a linking sequence of 0-50 amino acid residues to an HA1 C-terminal stem segment, comprising the amino acids from position y to and including the C-terminal amino acid of the HA1 domain; and (b) an influenza hemagglutinin HA2 domain, wherein the hemagglutinin stem domain polypeptide is resistant to protease cleavage at the junction between HA1 and HA2, and wherein one or more amino acid of the amino acids at positions 337, 340, 352, 353, 402, 406, 409, 413 and / or 416 have been mutated, as compared to the corresponding positions in wild-type influenza HA.
Owner:JANSSEN VACCINES & PREVENTION BV
Anaerobic enrichment medium and preparation method thereof
ActiveCN104419744APromote growthPromote reproductionMicrobiological testing/measurementBiotechnologyAnaerobic bacteria
The invention belongs to the technical field of microbial culture, and concretely relates to an anaerobic enrichment medium and a preparation method thereof. The medium includes peptone, yeast extract powder, a brain and heart infusion broth, glucose, sodium thioglycolate, hemin, vitamin K1, an anticoagulant, an anaerobic indicator and distilled water; and the medium for promoting mass propagation of anaerobic bacteria can be prepared through a special ratio of the above components. The medium is used to clinically culture anaerobic pathogens in order to improve the positive detection rate of the anaerobic pathogens.
Owner:SHANDONG XINKE BIOLOGICAL TECH
Preparation method of anti-avian influenza virus, newcastle disease virus, chicken bursa virus specific transfer and antimicrobial peptide
InactiveCN101684140AImprove immunityPeptide preparation methodsCytokines/lymphokines/interferonsFreeze thawingAntimicrobial peptides
The invention discloses a preparation method of anti-avian influenza virus, newcastle disease virus, chicken bursa virus specific transfer and antimicrobial peptide, which includes the steps of: (1) using avian influenza viral vaccine, newcastle disease virus vaccine, chicken bursa viral vaccine immune experiment pig; (2) closely monitoring vaccine immune antibody level of experiment pig in vivo,when the antibody reaches a peak value, sterilizedly fetching splenic organ or anticoagulated blood of experiment pig for preparing lymphocyte mono-layer; (3) executing subculture for lymphocyte mono-layer; (4) inducing and culturing the lymph subculture cell after which grows into a mono-layer with phaescolosaxin, then the anti-avian influenza virus, newcastle disease virus chicken bursa virus specific transfer and antimicrobial peptide are obtained. The product prepared by the method does not need to be purified further, and can be treated by ultrasonic and freeze thawing simple process intoa mixer for directly being used for bird in vivo, thereby playing roles for resisting avian influenza virus, newcastle disease virus, chicken bursa virus and bacteria, simultaneously improving immunity of bird.
Owner:TIANJIN SHENGJI GRP CO LTD
Medicine combination with function of improving nutritional anemia and preparation method thereof
The invention discloses a medicine combination with function of improving nutritional anemia and a preparation method thereof. The medicine combination is prepared by spirulina platensis phycocyanin, vitamin C, vitamin B2 and hemin according to certain weight percent. The medicine combination of the invention has the function of improving nutritional anemia.
Owner:BEIJING INCREASEPHARM CORP LTD
Use of hemagglutinin of the african swine fever virus as an adjuvant
InactiveUS20100086556A1Enhance immune responseHigh homologyOrganic active ingredientsVirusesHemagglutininAdjuvant
The invention generally relates to the use of the hemagglutinin (HA) of African swine fever virus (ASFV) as an adjuvant to enhance the immune response against an antigen in a subject. The invention provides a gene construct comprising all or part of the encoding sequence of said HA fused to the encoding sequence of an antigen. The invention is applicable in human and animal health.
Owner:FUNDACIO CENT DE RECERCA & SANITAT ANIMAL
Pseudotyped baculovirus to stimulate immunogenicity against avian influenza
The current invention relates to vaccines that use baculovirus vectors to expose a host organism to an immunogen, thereby eliciting an immune response. A pseudo-typed baculovirus is used to display hemagglutinin on the cell membrane in order to increase host immunogenicity.
Owner:NATIONAL TSING HUA UNIVERSITY
Monoclonal antibodies specific to hemagglutinin and neuraminidase from influenza virus h5-subtype or n1-subtype and uses thereof
ActiveUS20130004497A1High sensitivityStrong specificitySsRNA viruses negative-senseMicrobiological testing/measurementHemagglutininNeuraminidase
Monoclonal antibodies and related binding proteins that bind specifically to the envelope glycoprotein of H5 subtypes or neuraminidase glycoprotein of N1 subtypes of avian influenza virus (AIV) are provided. The monoclonal antibodies and related binding proteins are useful for the detection of H5 and N1 subtypes of AIV, including H5N1 subtypes and provide means for the diagnosis, surveillance and treatment of dangerous viral infections.
Owner:TEMASEK LIFE SCIENCES LABORATORY
Antibiotic-free composite premix feed for laying hens at laying period as well as preparation method and application of antibiotic-free composite premix feed
PendingCN106721026AQuality improvementImprove Gut HealthFood processingAnimal feeding stuffPhytaseAnimal science
The invention belongs to the technical field of feeds, and relates to an antibiotic-free composite premix feed for laying hens at a laying period, as well as a preparation method and the application of the feed. The feed consists of the following components in parts by weight: 5-10 parts of corn gluten meal, 8-25 parts of peanut meal, 5-10 parts of rice bran, 15-25 parts of sesame seed meal, 20-50 parts of dietary fibers, 1-10 parts of soybean oil, 5 parts of a 0.5% compound premix, 8-15 parts of stone powder, 8-15 parts of dicalcium phosphate, 2.5-4.5 parts of table salt, 1-3 parts of lysine, 1-3 parts of threonine, 1-3 parts of methionine, 0.1-0.5 parts of Nutri. P namely tannic acid, 1-3 parts of yeast hydrolyate, 0.3-2 parts of yeast cell wall powder, 0.1-0.5 part of selenium yeast, 0.1-0.5 part of zinc methionine, 0.2-0.5 part of hemoprotein, 0.2-0.5 part of a compound enzyme preparation, 0.8 part of choline chloride and 0.2 part of 10000 u / g phytase. Through the adoption of the antibiotic-free composite premix feed, the laying rate, the egg production and the egg weight can be notably increased.
Owner:LIAONING WELLHOPE AGRI TECH
Adenovirus vector avian influenza recombinant vaccine
ActiveCN101475641AImprove immunityGenetic material ingredientsAntiviralsProtective antigenHemagglutinin
The invention discloses an adenovirus carrier avian influenza recombined vaccine. The vaccine takes the hemagglutinin antigen gene of highly pathogenic avian influenza H5N1 as a main protective antigen gene which is fused with a braided mycobacterium tuberculosis heat shock protein coding gene. After being transferred into an adenovirus carrier to obtain a recombined adenovirus and immunize an animal, the fusion gene can produce a high titer antibody of the hemagglutinin antigen HA inside the animal and keep a high antibody titer inside the animal for a long period.
Owner:ZHEJIANG YEBIO BIOTECH +1
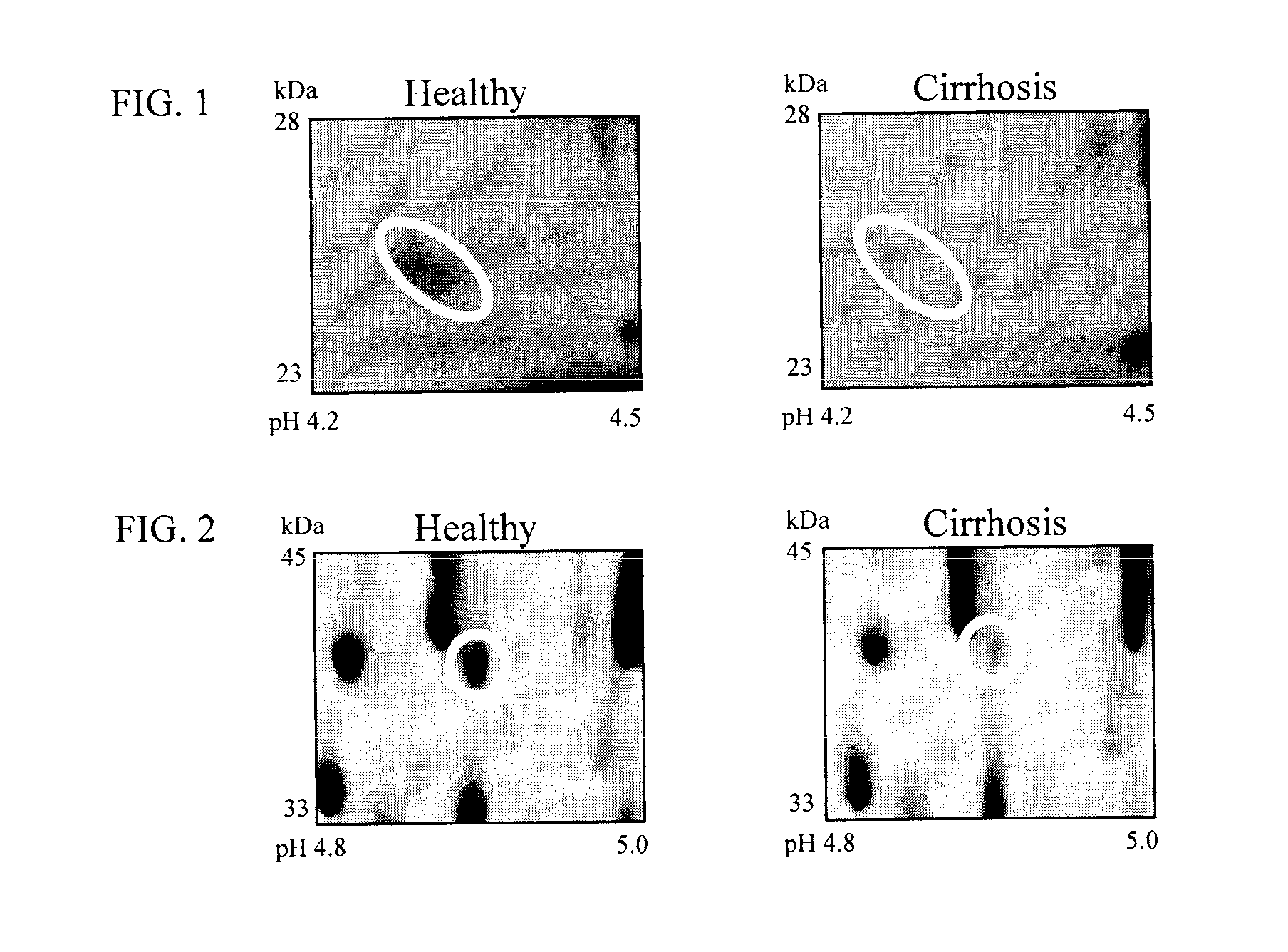
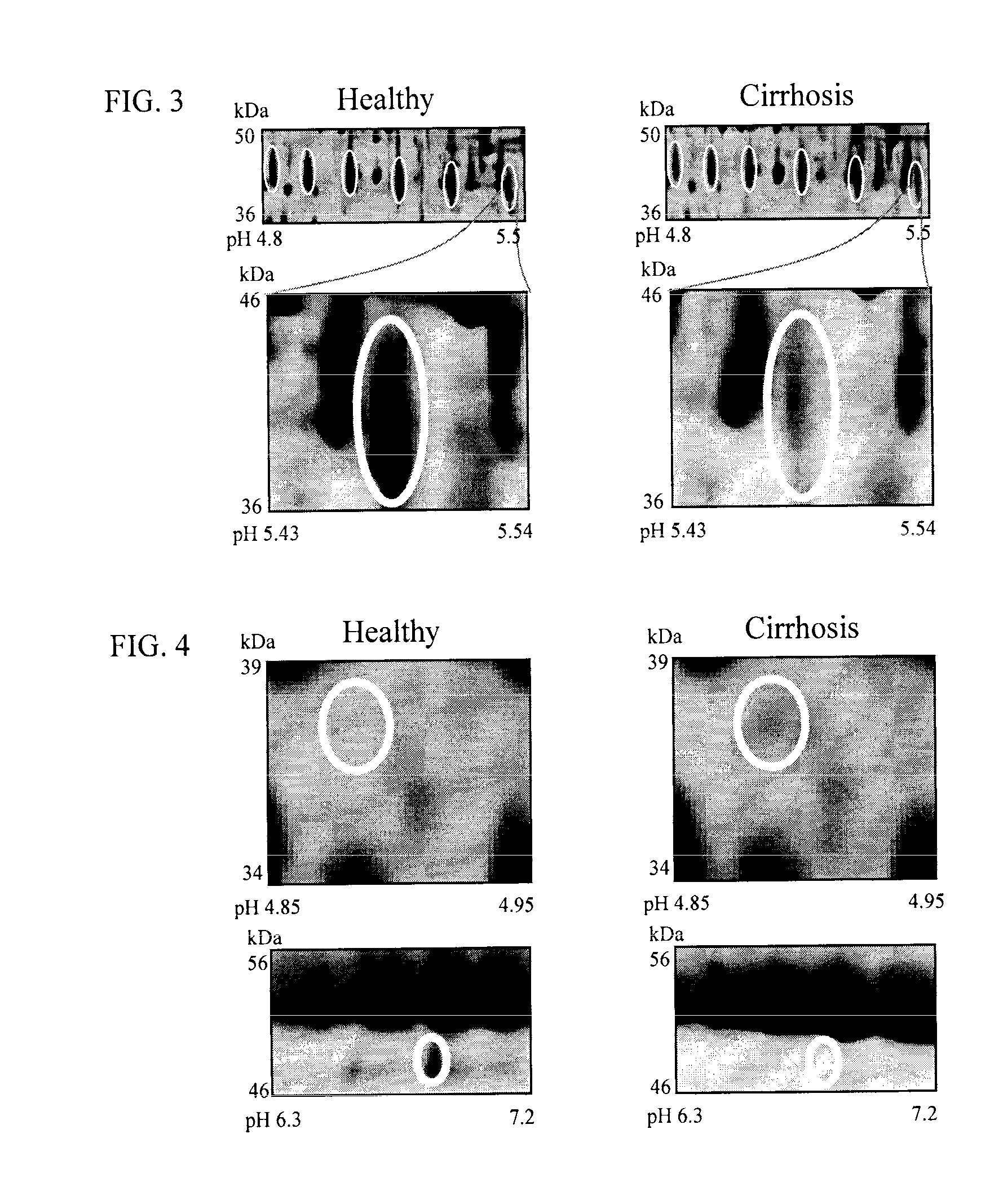
Clinical diagnosis of hepatic fibrosis using a novel panel of low abundant human plasma protein biomarkers
The inventors have proposed a novel panel of human plasma protein biomarkers for diagnosing hepatic fibrosis and cirrhosis. Presently there is no reliable non-invasive way of assessing liver fibrosis. A 2D-PAGE based proteomics study was used to identify potential fibrosis biomarkers. Plasma from patients with hepatic cirrhosis induced by infection with the hepatitis C virus (HCV) were analysed. Several proteins associated with liver scarring and potentially also related to viral infection were identified. These proteins include 14-3-3 protein zeta / delta, adiponectin, afamin, alpha-1-antitrypsin, alpha-2-HS-glycoprotein, apolipoprotein C-III, apolipoprotein E, C4b-binding protein beta chain, intact / cleaved complement C3dg, corticosteroid-binding globulin, fibrinogen gamma chain, beta haptoglobin at pH 5.46-5.49, haptoglobin-related protein, hemopexin, immunoglobulin J chain, leucine-rich alpha-2-glycoprotein, lipid transfer inhibitor protein, retinol-binding protein 4, serum paraoxonase / arylesterase 1, sex hormone-binding globulin and zinc-alpha-2-glycoprotein. These biomarkers can be used in conjunction with polypeptides in WO / 2008 / 031051. The concentrations of these novel biomarkers can be determined using an immunoassay where the concentrations would reflect the extent of fibrosis. A fibrosis scoring scale for each of the novel biomarkers is proposed. The additive result from the scores of all the novel biomarkers would give a more reliable indication of the degree of fibrosis rather than examining individual biomarkers.
Owner:UNIV OF OXFORD
Method for producing purified hematinic iron-saccharidic complex and product produced
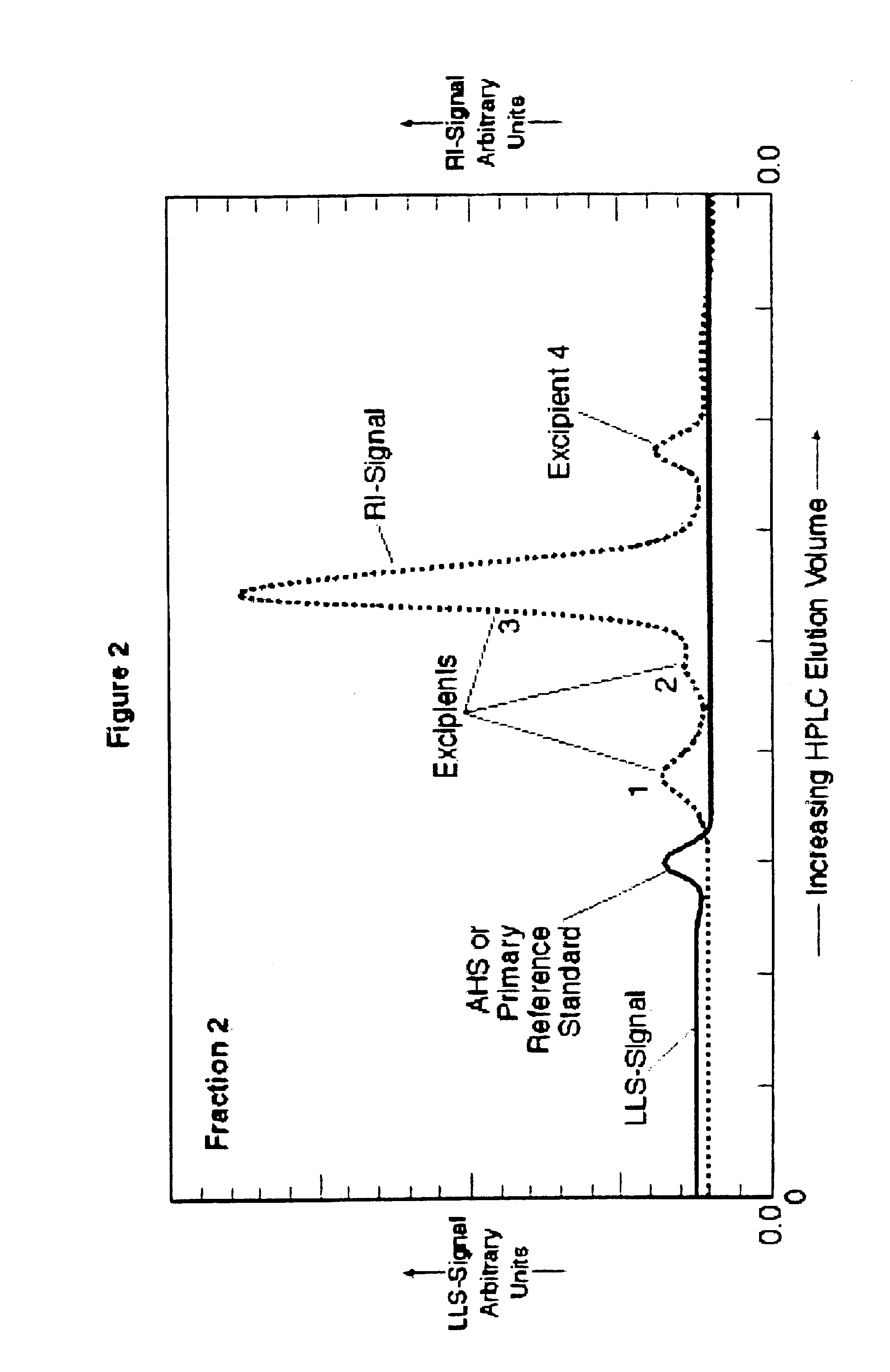
A method for separating and purifying the active hematinic species (AHS) present in iron-saccharidic compositions, including AHS such as sodium ferric gluconate complex, ferric hydroxide-sucrose complex and ferric saccharate complex and others of similar form and function. The method separates the AHS from one or more excipients and, preferably, lyophilizes the separated AHS. Separation of the AHS permits its analytical quantification, further concentration, purification and / or lyophilization as well as preparation of new and useful products and pharmaceutical compositions.
Owner:CHROMACEUTICAL ADVANCED TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com