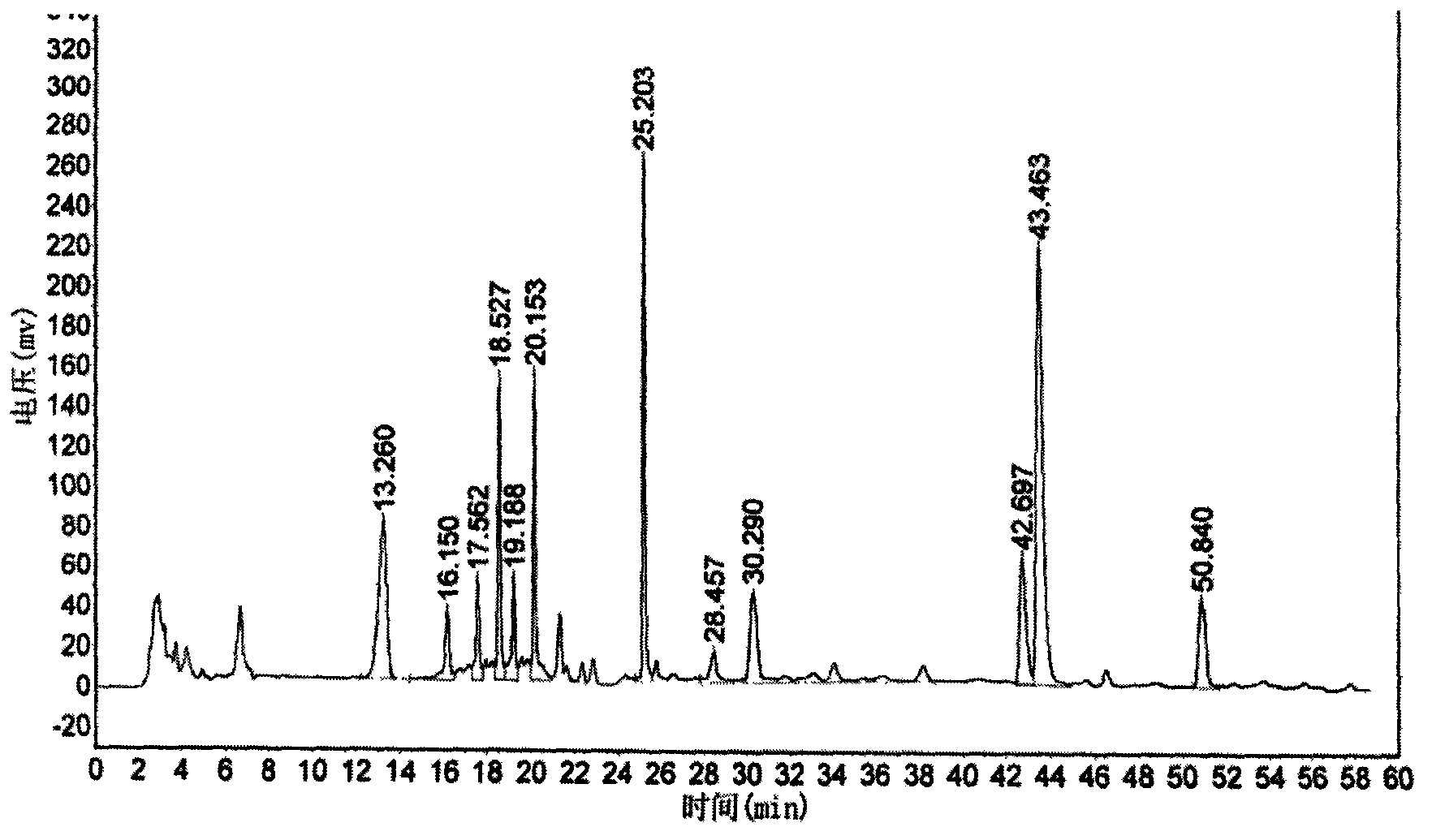
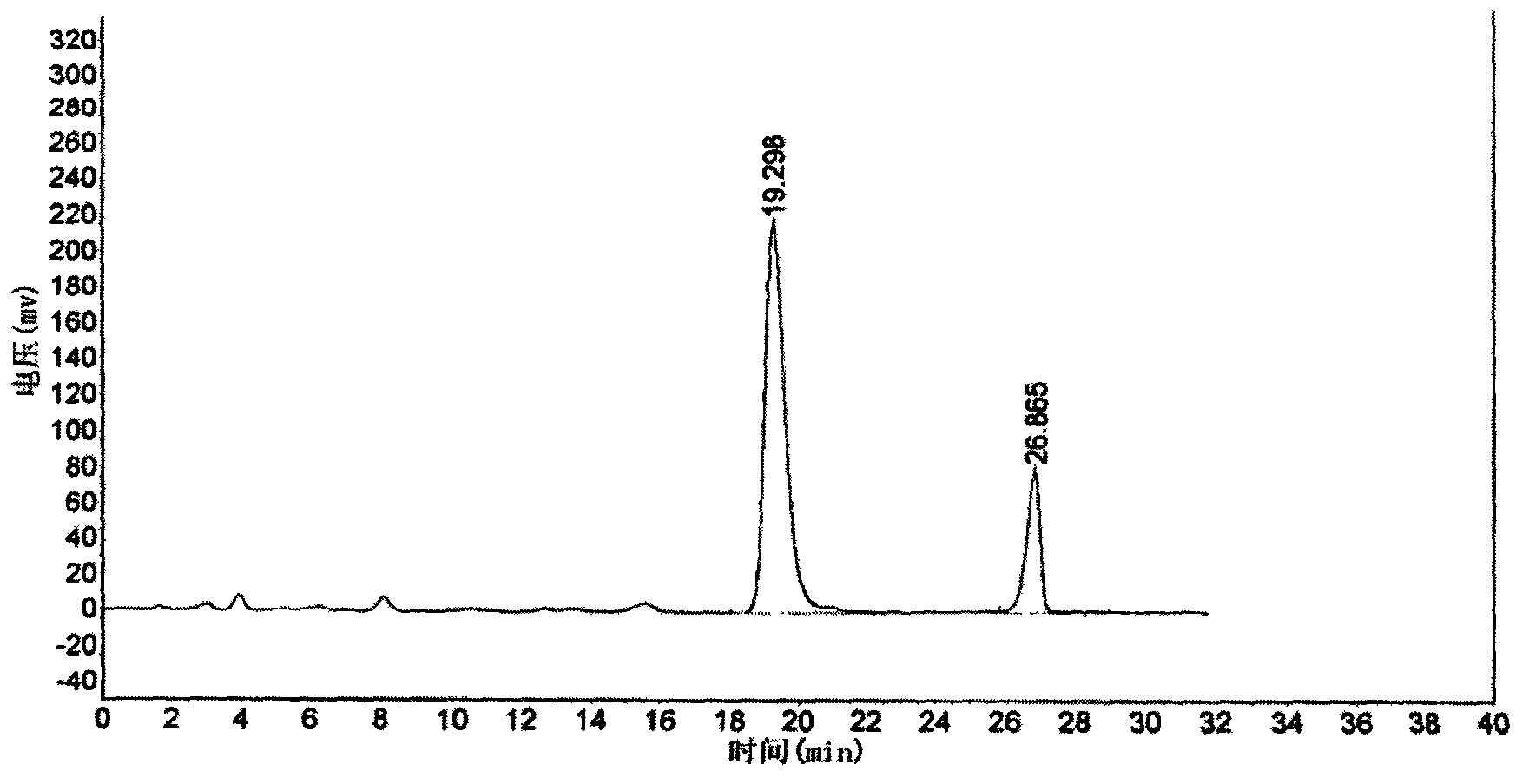
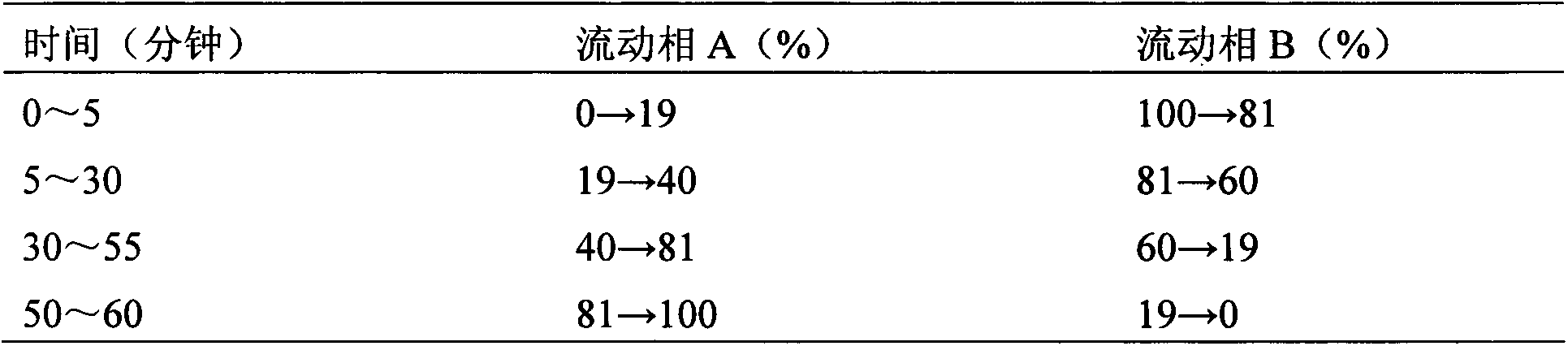
Six-flavor hematinic capsule, its quality control method and application thereof
A technology of Liuwei Buxue Capsules, which is applied in the field of Liuwei Buxue Capsules and its quality control, and can solve problems such as easy moisture absorption and hardening
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] (1) Weigh 680g of comfrey, 560g of white peony, 760g of angelica, 880g of rehmannia glutinosa, 420g of chuanxiong, 350g of astragalus, 5g of magnesium stearate, 48g of pregelatinized starch, 33g of microcrystalline cellulose, 10g of mannitol, and talcum powder 19g, silicon dioxide 5g, spare.
[0053] (2) Soak Angelica and Rhizoma Chuanxiong with 6 times the amount of water at 30°C for 1 hour, distill and extract the volatile oil for 6 hours, collect the volatile oil extract, store the distilled aqueous solution in another container for future use, and leave the residue for later use;
[0054] (3) Mix the medicinal dregs after extracting the volatile oil with Radix Paeoniae Alba, Comfrey, Radix Astragali, and Rehmannia glutinosa, add 10 times the amount of water to decoct twice, each time for 2 hours, filter in stages, combine the filtrate and the distilled aqueous solution and Concentrate to a clear paste with a relative density of 1.10 (45°C), stir and add 98% ethanol ...
Embodiment 2
[0060] (1) Weigh 680g of comfrey, 560g of white peony, 760g of angelica, 880g of rehmannia glutinosa, 420g of chuanxiong, 350g of astragalus, 5g of magnesium stearate, 48g of pregelatinized starch, 33g of microcrystalline cellulose, 10g of mannitol, and talcum powder 19g, silicon dioxide 5g, spare.
[0061] (2) Soak Angelica and Rhizoma Chuanxiong with 7 times the amount of water at 40°C for 1 hour, distill and extract the volatile oil for 5 hours, collect the volatile oil extract, store the distilled aqueous solution in another container for future use, and leave the residue for later use;
[0062] (3) Mix the medicinal dregs after extracting the volatile oil with Radix Paeoniae Alba, Comfrey, Radix Astragali, and Rehmannia glutinosa, add 11 times the amount of water to decoct twice, each time for 2 hours, filter in stages, combine the filtrate and the distilled aqueous solution and Concentrate to a clear paste with a relative density of 1.12 (50°C), stir when cooled to room ...
Embodiment 3
[0068] (1) Weigh 680g of comfrey, 560g of white peony, 760g of angelica, 880g of rehmannia glutinosa, 420g of chuanxiong, 350g of astragalus, 5g of magnesium stearate, 48g of pregelatinized starch, 33g of microcrystalline cellulose, 10g of mannitol, and talcum powder 19g, silicon dioxide 5g, spare.
[0069] (2) Soak Angelica and Rhizoma Chuanxiong with 8 times the amount of water at 50° C. for 1 hour, distill and extract the volatile oil for 4 hours, collect the volatile oil extract, store the distilled aqueous solution in another container for future use, and leave the residue for later use;
[0070](3) Mix the medicinal dregs after extracting the volatile oil with Radix Paeoniae Alba, Comfrey, Radix Astragali, and Rehmannia glutinosa, add 12 times the amount of water to decoct twice, each time for 2 hours, filter in stages, combine the filtrate and the distilled aqueous solution and Concentrate to a clear paste with a relative density of 1.15 (55°C), stir when cooled to room...
PUM
| Property | Measurement | Unit |
|---|---|---|
| recovery rate | aaaaa | aaaaa |
| recovery rate | aaaaa | aaaaa |
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com