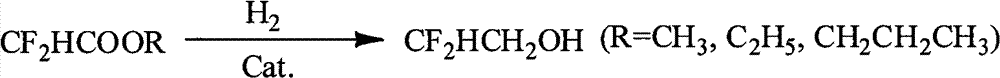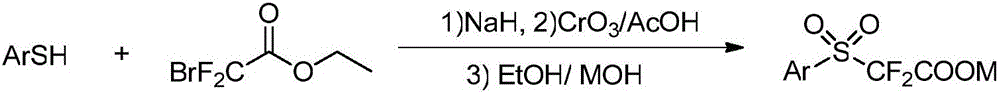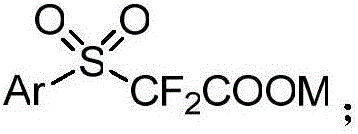Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
142 results about "Difluoroacetic acid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Difluoroacetic acid is a chemical compound with formula CHF₂COOH. It is a dihalogenocarboxylic acid, specifically a structural analog of acetic acid with two of three hydrogen atoms on the alpha carbon replaced with fluorine atoms. In solution, it dissociates to form difluoroacetate ions. Difluoroacetic acid can also be used as direct C-H difluoromethylating reagent.
Processing technique of ethyl difluoroacetate
InactiveCN102311343AHigh yieldHigh product yieldOrganic compound preparationCarboxylic acid esters preparationPotassium fluorideAmination
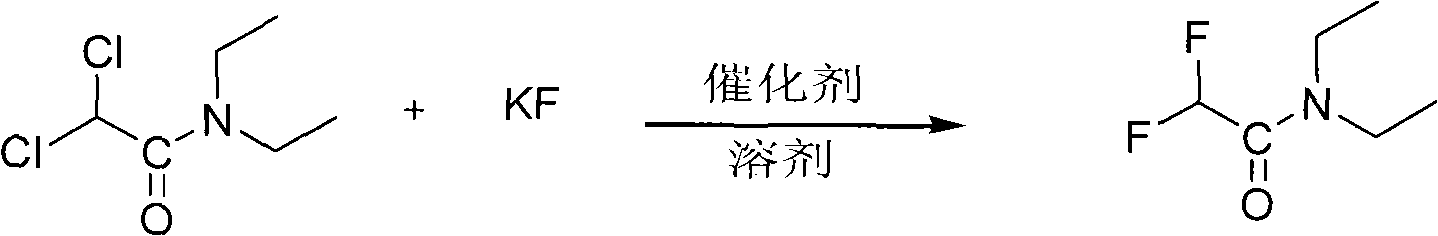
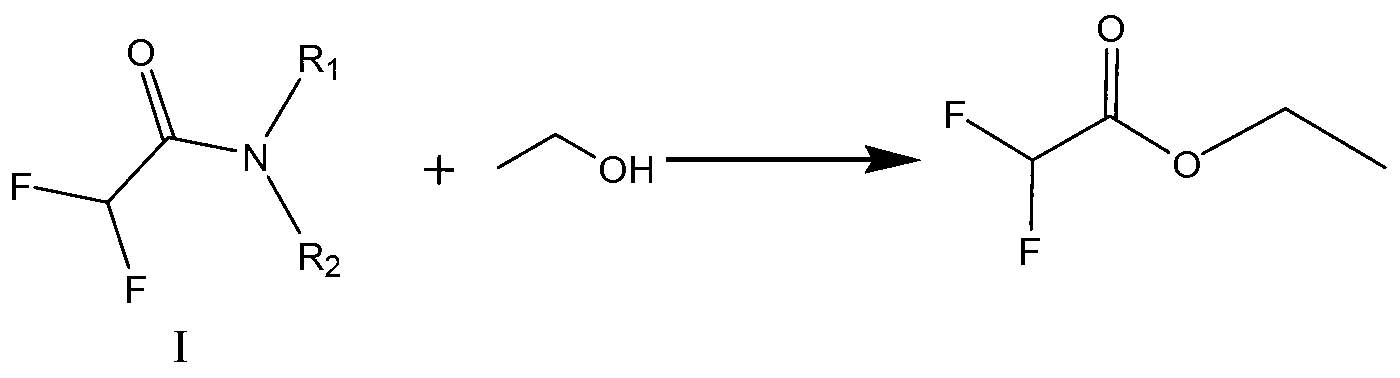
The invention mainly discloses a processing technique of ethyl difluoroacetate, which mainly comprises the following steps of: preparing an intermediate product dichloroacetyl diethylamine through an amination process; carrying out fluorination with anhydrous potassium fluoride and the dichloroacetyl diethylamine as raw material under the action of a solvent and a phase transfer catalyst to prepare difluoro-acetyl diethylamine; and carrying out esterification to prepare the ethyl difluoroacetate. The processing technique of the ethyl difluoroacetate is characterized in that the solvent in the fluorination is sulfolane, and the esterification is a direction reaction of ethanol and the difluoro-acetyl diethylamine. The processing technique of the ethyl difluoroacetate specifically has the advantages of improving the final yield by changing the solvent during the fluorination and improving the production rate by carrying out the direct esterification after the fluorination.
Owner:RUGAO JINLING CHEM
Industrialized synthetic method of ethyl difuoroacetate
InactiveCN102875379ASolve the problem that the fluorination reaction cannot occurOrganic compound preparationCarboxylic acid esters preparationAlcoholDifluoroacetic acid
The invention discloses an industrialized synthetic method of ethyl difluoroacetate. Difluoroacetate is generated through catalysis of difluoroacetamide and low alcohols under strong acid, and the selectivity of the difluoroacetate reaches more than 95%. The method is characterized in that dichloro-acetamide is prepared from secondary amide that is easy to recover and can be in continuous operation at the same time, the problem that fluorine substitution reaction is difficult to carry out under the combination of a common solvent and a catalyst is solved through a solvent, and the solvent has the effect of a phase transfer agent, and is low in cost and convenient to recover.
Owner:上海品沃化工有限公司
Preparation method of 2-bromo-2,2-difluoroacetyl chloride and 2-bromo-2,2-difluoro acetate and recycling method of waste difluoro trichloroethane
InactiveCN104761446AEnable recyclingIncrease added valuePreparation by hydrogen halide split-offPreparation from carboxylic acid halidesBromoethanePhenol
The invention relates to a preparation method of 2-bromo-2,2-difluoroacetyl chloride and 2-bromo-2,2-difluoro acetate and a recycling method of waste difluoro trichloroethane. The preparation method comprises the following steps: with waste difluoro trichloroethane produced in the production process of dichlorotrifluoroethane as a raw material, carrying out dehydrochlorination to obtain difluoro dichloroethylene, carrying out addition reaction on difluoro dichloroethylene and bromine to obtain difluoro dichlone dibromoethane; reacting difluoro dichlone dibromoethane with sulfur trioxide to obtain 2-bromo-2,2-difluoroacetyl chloride; and reacting 2-bromo-2,2-difluoroacetyl chloride with alcohol or phenol to obtain 2-bromo-2,2-difluoro acetate series products. According to the preparation method and the recycling method, recycling of waste difluoro trichloroethane is realized; 2-bromo-2,2-difluoroacetyl chloride and 2-bromo-2,2-difluoro acetate are prepared by a temperature oscillation method, so that the production cost is reduced; and meanwhile, the preparation method is an environment-friendly technique for producing products.
Owner:JIANGXI SUNWAY CHEM CO LTD
Method for preparing difluoroethanol
ActiveCN102766024AHigh reaction yieldShort reaction timeOrganic compound preparationHydroxy compound preparationHydrogen pressureDifluoroacetic acid
The invention discloses a method for preparing difluoroethanol and aims at solving problems of long reaction time and low yield. Difluoro-acetic acid ester serves as the raw material, and the method includes the following steps: adding catalysts, difluoro-acetic acid ester and ethanol into a reaction kettle, leading hydrogen pressure to be 5-10MPa, stirring to lead temperature to rise to 180-250 DEG C, and reacting for 6-10h to obtain difluoroethanol, wherein mass ratio of catalysts: difluoro-acetic acid ester: ethanol is 1: (6.25-12.5):(12.5-18.75). According to mass percent, the catalysts are composed of 40-70% of copper oxide, 10-50% of aluminum oxide, 5-30% of manganese oxide and 5-30% of barium monoxide. The method is mainly used for preparing difluoroethanol.
Owner:XIAN MODERN CHEM RES INST
Method for preparing difluoroacetic acid and salts thereof
InactiveCN101977885APreparation from carboxylic acid halideCarboxylic acid halides preparationHydrofluoric acidDifluoroacetic acid
The invention relates to a method for preparing difluoroacetic acid and the salts thereof. The invention also relates to the preparation of difluoroacetyl fluoride used as an intermediate product in the preparation of difluoroacetic acid. The method for preparing difluoroacetic acid according to the invention is characterised in that the same comprises the step of preparing difluoroacetyl fluoride by reacting dichloroacetyl chloride with hydrofluoric acid in a gaseous phase and in the presence of a chromium-based catalyst, followed by the step of hydrolysing the difluoroacetyl fluoride thus obtained.
Owner:RHODIA OPERATIONS SAS
Preparation method for difluoro acetate
InactiveCN101270050AHigh yieldHigh purityOrganic compound preparationCarboxylic acid esters preparationAlcoholDifluoroacetic acid
The present invention discloses a preparation method of difluoro acetate. In the method, difluoro acetonitrile is used as raw material and reacts with alcohol and water to further synthesize the difluoro acetate with a catalyst; the temperature of the synthesis is between 0 DEG C and the reflux temperature of the reaction solution; the molar ratio of the difluoro acetonitrile, the catalyst, the alcohol and the water is equal to 1 : 0.5 to 10.0 : 0.5 to 10.0 : 0.5 to 5.0; the reaction solution can be refined to prepare the difluoro acetate, or extracted, delaminated and refined to prepare the difluoro acetate. The method can be used for preparing the difluoro acetate with high yield rate and high purity. With an appropriate ratio, the transformation ratios of the raw materials exceed 99.0 percent; the selectivity of various esters can exceed 95.0 percent; the yield rate is more than 85 percent. The purity of the synthesized product is more than or equal to 99.5 percent and is suitable for the needs in various aspects. The method has the advantages of short technical process, simple operation, high yield, less three-waste, high purity of the product and so on.
Owner:JUHUA GROUP TECH CENT +1
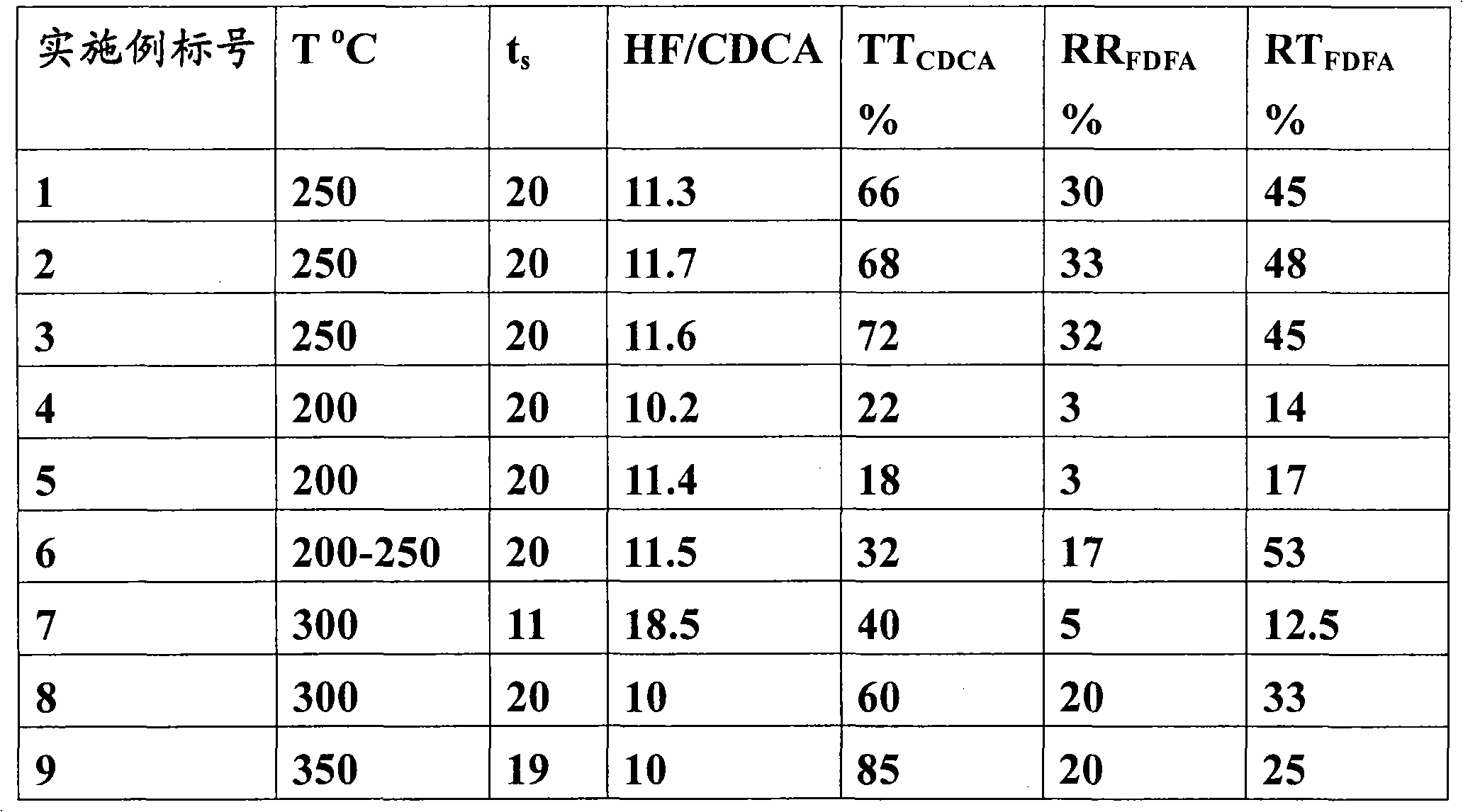
Method for preparing difluoroethanol by gas phase catalytic hydrogenation
ActiveCN103524306AImprove conversion rateGood choiceOrganic compound preparationHydroxy compound preparationHydrogenGas phase
The invention discloses a method for preparing difluoroethanol by gas phase catalytic hydrogenation. The method comprises the following steps: in the presence of a catalyst, mixing difluoroacetate and hydrogen in a molar ratio of 1:(1-10); carrying out a reaction under conditions that the reaction temperature is 100-400 DEG C, the reaction pressure is ordinary pressure and the reaction idling speed is 1-100 / s<-1>; and collecting a reaction product and rectifying to obtain the difluoroethanol. The method disclosed by the invention has the advantages of mild reaction temperature, high raw material conversion ratio, good product selectivity and low cost.
Owner:ZHEJIANG JUHUA HANZHENG NEW MATERIAL +1
Method for synthesizing ethyl 4,4-difluoroacetoacetate in catalyzed mode through layered materials
ActiveCN105001085AImprove conversion rateHigh yieldPhysical/chemical process catalystsOrganic compound preparationSolventEthyl acetate
The invention discloses a method for synthesizing ethyl 4,4-difluoroacetoacetate in a catalyzed mode through layered materials. The method includes the following steps: 1, a divalent and trivalent metal salt solution and a sodium hydroxide and sodium carbonate mixed solution are rapidly mixed to prepare multielement hydrotalcite, the multielement hydrotalcite is roasted to be added into a fluoride-salt saturation aqueous solution, the mixture is stirred, filtered, washed and dried to be roasted under the nitrogen condition, and catalysts are obtained; 2, ethyl acetate, ethyl difluoroacetate and the catalysts are added into a four-opening flask, an ethyl alcohol and ethyl acetate mixture is evaporated after temperature rising, stirring and reacting are carried out, cooling is carried out, catalysts are filtered and recycled, filtrate is acidized, cooled and filtered, and pressure reduction rectification is carried out on the filtrate to obtain the ethyl 4,4-difluoroacetoacetate; 3, the recycled catalysts are sufficiently washed through solvents and roasted to be added into a fluoride-salt saturation aqueous solution, the mixture is stirred, filtered, washed and dried to be roasted under the nitrogen condition, and the catalysts are regenerated. The method has the advantages that the yield is high, the catalysts can be recycled, and the method is safe and environmentally friendly.
Owner:QUZHOU UNIV +1
Process for preparing bromodifluoacetic acid compounds
InactiveCN1392133APreparation from carboxylic acid halidePreparation from carboxylic acid halidesOleumAlcohol
The invention relates to a method for preparing bromodifluoroacetic compounds which comprises converting a 1,1-diflouro-1,2-dibromodihaloethane with oleum having 50-70% SO3 to a bromodifluoroacetyl halide (bromide or chloride) and then in reacting the latter directly with either an alcohol, or with water.
Owner:ATOFINA
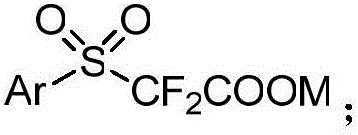
Aryl sulfuryl difluoroacetic salt compound, as well as preparation method and application thereof
ActiveCN106748921AMild reaction conditionsReduce manufacturing costOrganic compound preparationSulfide preparationArylAlcohol
The invention discloses an aryl sulfuryl difluoroacetic salt compound, as well as a preparation method and application thereof. The aryl sulfuryl difluoroacetic salt compound reacts with aldehyde at room temperature to obtain an aryl sulfuryl substituted alpha-difluoromethyl alcohol compound. The aryl sulfuryl difluoroacetic salt compound has the beneficial effects of mild reaction condition, high product yield, few side effect and high universality when the aryl sulfuryl difluoroacetic salt compound is adopted to prepare the aryl sulfuryl substituted alpha-difluoromethyl alcohol compound.
Owner:CHINA GATEWAY PHARMA DEV CO LTD
Method for preparing difluoroacetic acid esters
InactiveCN102471217AOptimal implementation methodPreparation from carboxylic acid halidesOrganic compound preparationAlcoholDifluoroacetic acid
The present invention relates to a method for preparing difluoroacetic acid esters. The method of the invention for preparing difluoroacetic acid esters is characterized in that it includes reacting difluoroacetyl fluorine with an aliphatic or cycloaliphatic alcohol in the presence of a heterogeneous mineral base.
Owner:RHODIA OPERATIONS SAS
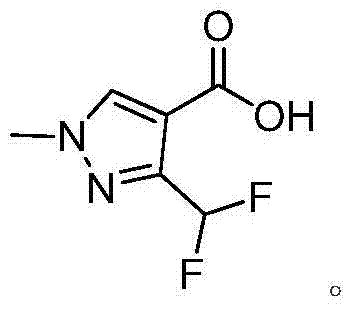
Preparation method of 3-(difluoromethyl)-1-methyl-1H-pyrazol-4-carboxylic acid
The invention discloses a preparation method of 3-(difluoromethyl)-1-methyl-1H-pyrazol-4-carboxylic acid. The reaction formula is shown in the specification. Difluoroacetic acid, which is relatively low in price and small in molar mass and is introduced at a post-synthesis phase is adopted, so that the preparation method of the 3-(difluoromethyl)-1-methyl-1H-pyrazol-4-carboxylic acid is low in cost, simple and safe in technological operation, high in product content, and high in product yield; the content is 98.1-98.9% (liquid chromatogram, external standard); the total yield is 52.2-56.9% (based on propargyl alcohol). Nanoscale titanium dioxide is adopted to carry out catalytic esterification, so that the reaction yield is high; the reaction time is short; the used raw materials and solvent are cheap and available; furthermore, an organic solvent is avoided in the four reaction steps, so that the solvent recovery cost and the environmental pollution risk are greatly reduced.
Owner:HUNAN CHEM RES INST
Method for preparing ethyl bromodifluoroacetate
InactiveCN107573242AAvoid damageLess investmentOrganic compound preparationCarboxylic acid esters preparationCupric bromidePotassium fluoride
The invention provides a method for preparing ethyl bromodifluoroacetate and relates to a preparation method of a chemical reagent. The method takes trichloro ethylene as a raw material, and comprisesthe following steps: under the action of ultraviolet light of a catalytic medium, an oxidation reaction happens between trichloro ethylene and oxygen in a reactor to synthesize dichloracetyl chloride; an amination reaction happens between dichloracetyl chloride and diethylamine under the action of a catalyst to synthesize dichloroacetyl diethylamine; a fluorination reaction happens between dichloroacetyl diethylamine and anhydrous potassium fluoride under the action of a solvent and a phase transfer catalyst to synthesize difluoroacetyl diethylacetamide; difluoroacetyl diethylacetamide is esterified to synthesize ethyl difluoroacetate; and by taking cupric bromide as a brominating agent, ethyl difluoroacetate is bromized to prepare the end product (ethyl bromodifluoroacetate). The methodhas the characteristics that the equipment investment is low, reaction conditions are mild, the method is safely implemented at normal pressure, and after-treatment is simple.
Owner:盐城顺恒化工有限公司
Resource utilization method of tail gas from trifluoroethylene production
InactiveCN102863313AHas the effect of energy saving and emission reductionSimple processPreparation by halogen additionEthyl acetatePlant growth
The invention discloses a resource utilization method of tail gas from trifluoroethylene production. According to the invention, addition reaction between the tail gas from the trifluoroethylene production and bromine is performed to generate dibromotrifluoroethane and dibromotrifluoro chloroethane. The preparation method has the following advantages: the technology is simple; reaction condition is mild; purification is easy; and the technology is suitable for large-scale production. The method provided by the invention has effects of energy conservation and emission reduction. The prepared dibromotrifluoroethane can be used to prepare a dry etching gas hexafluorobutadiene, novel gyroscope suspension fluorobromohydrocarbon oil, alpha, beta, beta-trifluorostyrene and an insecticide and acaricide 1,4-diaryl-2,3-difluoro-2-butene. The prepared dibromotrifluoro chloroethane can be used to prepare a plant growth regulator's intermediate ethyl bromodifluoroacetate and a chain transfer agent used as radical polymerization.
Owner:SINOCHEM LANTIAN +1
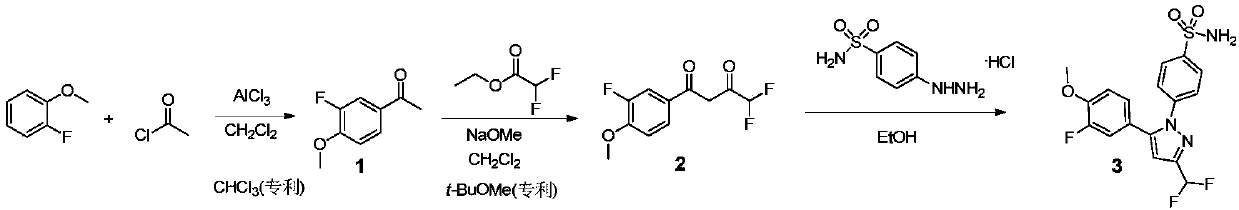
Preparation method of Deracoxib
The invention discloses a preparation method of Deracoxib, and belongs to the field of Deracoxib preparation. The preparation method of Deracoxib disclosed by the invention comprises the following steps: (1) taking methane chloride as a reaction solvent, and reacting 2-fluoroanisole with acetylchloride under the effect of acid so as to obtain 3-fluoro-4-methoxyacetophenone; (2) taking methane chloride as a reaction solvent, and reacting 3-fluoro-4-methoxyacetophenone with ethyl difluoroacetate under the effect of alkaline so as to obtain 4,4-difluoro-1-(3-fluoro-4-methoxyphenyl)-1,3-butanedione; and (3) under the existence of an ethyl alcohol solvent, reacting 4,4-difluoro-1-(3-fluoro-4-methoxyphenyl)-1,3-butanedione with para-sulfamine phenylhydrazine or salt thereof so as to obtain Deracoxib. According to the preparation method of Deracoxib disclosed by the invention, dichloromethane is used as a solvent, the toxicity of the solvent is low, the cost of the solvent is lower than thatof methyl tertiary butyl ether, and the production cost is obviously reduced on the premise of guaranteeing the yield.
Owner:NORTHEAST AGRICULTURAL UNIVERSITY
Fluorine-containing phenanthridine derivative and preparation method thereof
ActiveCN105348194AMild responseSimple and fast operationOrganic chemistryDifluoroacetic acidPotassium
The invention relates to a fluorine-containing phenanthridine derivative and a preparation method thereof. The structure of the derivative is as shown in the specification. In the structure, R1 is -H, -F or -Cl; R2 is -H or -CH3; and R3 is -H, -CH3 or -OMe. According to the invention, under the condition of silver catalysis, potassium difluoroacetate undergoes decarboxylation and then is coupled with o-phenylisonitrile, so synthesis of a fluorine-containing phenanthridine heterocyclic compound is completed.
Owner:SHANGHAI UNIV
Process for preparing 4.4-difluoro-2-amino butyric acid with optical activity and ester thereof
InactiveCN1990458AReasonable choice of reaction processLower synthesis costOrganic compound preparationAmino-carboxyl compound preparationOxygenHydrolysis
The invention relates to a method for preparing chiral alpha- aminobutyric acid with difluorobenzyl methyl function group, especially a method for preparing 4, 4- difluorobenzyl- 2- amino- butyrate and the relative ester. The invention solves problems of dear agent and low productivity in current process. The method comprises following steps: reducing difluorobenzyl acetate to get hemiacetal, catalyzing hemiacetal with organic acid and reacting with ylide phosphorous salt to get difluorobenzyl crotonic ester, hydrolyzing difluorobenzyl crotonic ester under basic condition and getting difluorobenzyl butenoic acid, catalytic hydrogenating difluorobenzyl butenoic acid and getting 4, 4- difluorobenzyl butyrate; directing 4, 4- difluorobenzyl butyrate with Evans ((S)-4- R2- 1, 3- oxygen nitrogen- zole- dione or (R)- 4- R2-1, 3- oxygen nitrogen- zole- dione ) to get chiral azides; hydrogenrating and protecting with amnio said azides and removing directing group from it and getting 4, 4- difluorobenzyl- 2- amino- butyrate of optical-purity; getting relative ester through normal deravitive reaction.
Owner:上海药明康德新药开发有限公司
Preparation method of ethyl difluoroacetate and intermediate thereof
ActiveCN103254074AShort processWaste very littleOrganic compound preparationCarboxylic acid esters preparationPotassium fluorideDifluoroacetic acid
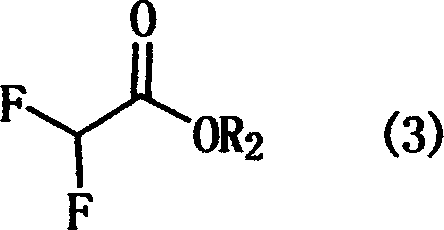
The invention discloses a preparation method of ethyl difluoroacetate and an intermediate thereof. The preparation method comprises the following steps of: dropwise adding a mixed solution of a compound I and ethanol to concentrated sulfuric acid at a temperature in the range from 90 to 110 DEG C for reacting, and simultaneously, distilling out generated ethyl difluoroacetate in the dropwise adding process, wherein each of R1 and R2 independently represents phenyl or C1-3 linear-chain or branch-chain alkyl, respectively and when each of R1 and R2 independently is the C1-3 linear-chain or branch-chain alkyl, R1 and R2 are different; and the preparation of the compound I comprises the steps of mixing a compound II with potassium fluoride for reacting in the presence of a catalyst namely a calixarene compound in a solvent; and the preparation of the intermediate compound II comprises the steps of mixing a compound III with a solvent, and dropwise adding dichloroacetyl chloride at a temperature in the range from -15 to 150 DEG C for reacting. The preparation method disclosed by the invention employs dichloroacetyl chloride and secondary amine as starting raw materials, and is simple and convenient in production process, low in quantity of three wastes, high in yield and suitable for industrial production.
Owner:JIANGSU LIANHE CHEM TECH +4
Preparation technique of ethyl difluoroacetate
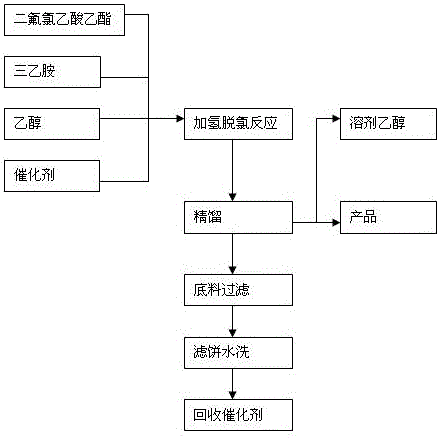
ActiveCN105859553AEasy to makeHigh purityOrganic compound preparationCarboxylic acid esters preparationFiltrationHigh pressure
The invention discloses a preparation technique of ethyl difluoroacetate. The preparation technique comprises the following steps: sequentially adding halogenated ethyl difluoroacetate, triethylamine, a solvent and a catalyst into a high-pressure reaction kettle; replacing air in the reaction kettle with nitrogen, replacing with hydrogen, and heating to perform hydrogenation reaction until the kettle pressure reaches the standard value; when the kettle pressure drops, repeatedly introducing hydrogen until the kettle pressure no longer drops; and finally, carrying out rectification reaction, carrying out normal-pressure rectification operation on the obtained product in a glass bottle to separate out the product ethyl difluoroacetate, and carrying out filtration and water washing on the kettle bottom material to recover the catalyst for the reaction next time. By adopting the halogenated ethyl difluoroacetate as the raw material, the preparation technique of ethyl difluoroacetate has the advantages of simple preparation process, high product purity (at least 99.5%) and high yield (at least 95%), and the catalytic efficiency of the palladium-carbon catalyst does not obviously descend after the palladium-carbon catalyst is recovered 10 times, thereby enhancing the benefits of the enterprise.
Owner:NANTONG BAOKAI CHEM
Preparation method of methyl difluoroacetate
InactiveCN102531895APhysical/chemical process catalystsOrganic compound preparationOrganic solventDifluoroacetic acid
The invention relates to a method for preparing difluoromethyl acetate. The method is characterized by comprising the following steps of: mixing methyl dichloroacetate with potassium fluoride serving as a catalyst; reacting at a high temperature; slowly adding an organic solvent; cooling a system to be below zero DEG C and preserving heat; stirring; distilling under reduced pressure; filtering; washing a precipitate with methylbenzene; continually drying a filtrate with anhydrous sodium sulfate; and driving a filter cake away to obtain difluoromethyl acetate serving as a product.
Owner:SHANGHAI SINOFLUORO SCI
Method for preparing difluoroacetic acid and salts thereof
InactiveUS8299300B2Preparation from carboxylic acid halideOrganic compound preparationHydrofluoric acidDifluoroacetic acid
The invention relates to a method for preparing difluoroacetic acid and the salts thereof. The invention also relates to the preparation of difluoroacetyl fluoride used as an intermediate product in the preparation of difluoroacetic acid. The method for preparing difluoroacetic acid according to the invention is characterized in that the same comprises the step of preparing difluoroacetyl fluoride by reacting dichloroacetyl chloride with hydrofluoric acid in a gaseous phase and in the presence of a chromium-based catalyst, followed by the step of hydrolysing the difluoroacetyl fluoride thus obtained.
Owner:RHODIA OPERATIONS SAS
Method for catalytically hydrogenating difluoroacetic acid
InactiveCN102531843AOrganic compound preparationHydroxy compound preparationSodium bicarbonateOrganic solvent
The invention relates to a method for catalytically hydrogenating difluoroacetic acid. The new method includes the following steps: step 1: preparation of catalyst: sodium borohydride as catalyst is added into organic solvent solution; step2: implementation of main reaction: the organic solvent with the sodium borohydride is added into a reaction kettle, the reaction kettle is sealed, temperature is increased, the difluoroacetic acid is added, and after sufficient reaction, the temperature is kept; step 3: extraction and rectification of product: the reaction product in step 2 is added with 300ml of water, the organic solvent is recovered by distillation, hydrochloric acid is used for regulating the pH value to 2 to 3, sodium bicarbonate solution is then used for regulating the pH value to 10, and after filtration and drying, product is obtained.
Owner:SHANGHAI SINOFLUORO SCI
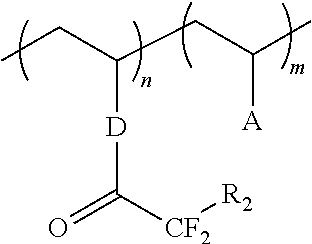
Superacid functional compounds
ActiveUS20130131201A1Organic compound preparationIon-exchanger regenerationGrignard reagentSynthesis methods
The invention relates to a novel synthesis method for forming superacid functional molecules that include monomers, as well as new polymers and copolymers formed from the monomers, and uses for these superacid molecules, polymers, and copolymers. The superacid molecules have an alpha,alpha-difluorosulfonic acid functionality that can be obtained by a reaction between various Grignard reagents and an alkyl(2-fluorosulfonyl)-1,1-difluoroacetate, such as methyl(2-fluorosulfonyl-1,1-difluoroacetate. The molecules, polymers and copolymers would be expected to have enhanced ion conductivity, and would be useful in a variety of applications, including as ion-conductive materials, surfactants, and ion exchange resins.
Owner:ARKEMA INC
Synthesizing method for ethyl difluoroacetate
InactiveCN105461560ASimplify production stepsImprove securityOrganic compound preparationCarboxylic acid esters preparationTube furnaceDifluoroacetic acid
The invention relates to a synthesizing method, in particular to a synthesizing method for ethyl difluoroacetate, and aims at solving the problems that an existing synthesizing method for the ethyl difluoroacetate is complex in technological process, low in safety and high in preparation cost. The synthesizing method comprises the steps that 1, a catalyst is prepared, wherein a tubular reactor is filled with Al2O3 powder with the certain particle size meshes, it is guaranteed that the height of the Al2O3 powder is in a heating area of the tubular furnace, the temperature is increased to 250 DEG C-300 DEG C under nitrogen protection and kept for 12 h, CHaFbClc gas is continuously and slowly introduced for a reaction, the reaction temperature is set at 250 DEG C-300 DEG C, and the reaction time is set for 12 h; 2, 1,1,2,2-tetrafluoroethyl ethylether is taken as raw materials for a reaction under the conditions of the certain temperature and the catalyst, and an intermediate does not need to be separated and is directly collected through condenser to be converted into ethyl ester. The method belongs to the field of chemical engineering.
Owner:TIANJIN CHANGLU CHEM NEW MATERIAL CO LTD
Preparation method of copper and zinc catalyst and application
ActiveCN106807384ALarge specific surface areaImprove conversion rateOrganic compound preparationHydroxy compound preparationRepeatabilityZinc salts
The invention provides a preparation method of a copper and zinc catalyst. The preparation method comprises the following steps of: mixing 2-13 parts by weight of soluble copper salt, 2-4 parts by weight of soluble zinc salt and 1-3 parts by weight of starch; performing primary grinding; then adding 2-8 parts by weight of sodium carbonate and 1-3 parts by weight of starch; performing secondary grinding; and washing, drying and calcining the mixture to obtain the copper and zinc catalyst. Compared with the prior art, by taking starch as a pore forming material, the specific surface area of the copper and zinc catalyst is effectively increased, and the copper and zinc catalyst is relatively high in catalytic activity and difluoroethanol selectivity. The preparation process is rapid and simple and good in repeatability. The invention also provides an application of the copper and zinc catalyst in hydrogenation preparation of difluoroethanol by methyl difluoroacetate. The catalyst is environmental-friendly and realizes green and environmental-friendly and energy-saving and emission-reducing effects; and moreover, the conversion rate of methyl difluoroacetate is relatively high, and the selectivity of difluoroethanol is good.
Owner:杭州催研科技有限公司
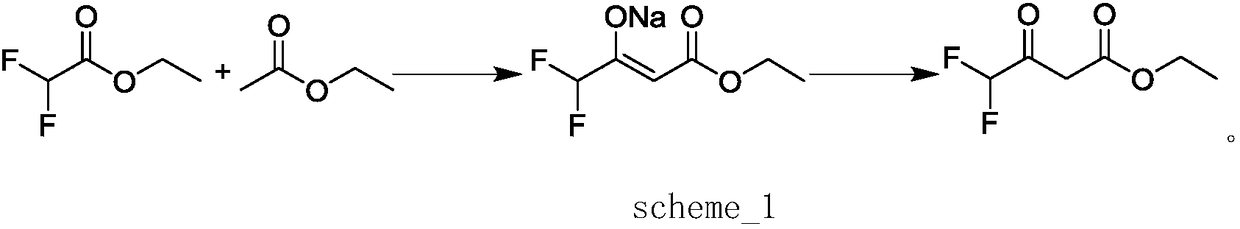
Method for preparing ethyl 4,4-difluoroacetoacetate
InactiveCN108218703AHigh yieldHigh selectivityOrganic compound preparationCarboxylic acid esters preparationAcetic acidOrganic solvent
The invention discloses a method for preparing ethyl 4,4-difluoroacetoacetate. The method comprises the following step: by taking ethyl difluoroacetate and ethyl acetate as raw materials, and an ethanol solution of sodium ethoxide as a catalyst, in the presence of an organic solvent, carrying out a Claisen condensation reaction, thereby obtaining the ethyl 4,4-difluoroacetoacetate. The method disclosed by the invention has the characteristics of being gentle in reaction condition, high in security, good in reaction selectivity, easy in product separation and purification, applicable to industrial production, and the like.
Owner:ZHEJIANG LANTIAN ENVIRONMENTAL PROTECTION HI TECH +1
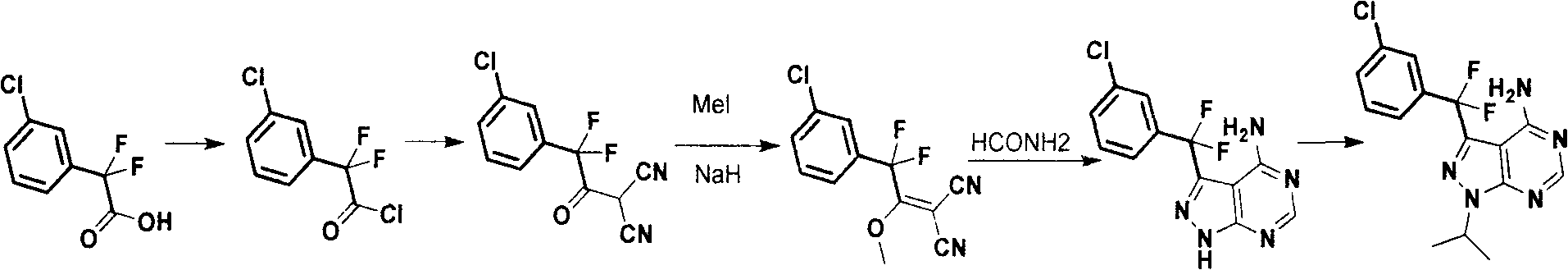
Synthesis process for anti-tumor new medicine 3-{(3-chlorphenyl)difluoromethyl-1-isopropyl-monohydro-pyrazolo(3,4-D)pyrimidine-4-amine compound
The invention discloses a synthesis method for an anti-tumor new medicine 3-{(3-chlorphenyl)difluoromethyl-1-isopropyl-monohydro-pyrazolo(3,4-D)pyrimidine-4-amine compound. The synthesis method comprises the following steps of: acylating 3-chlorphenyl-2 oxalic acid serving as an initiative raw material; reacting the acylated material with dipropionitrile; performing methylation; performing ring closure with hydrazine hydrate to obtain 5-amino-1-(3-chlorophenyl)-pyrazole-4-carbonitrile, crystallizing and purifying, heating and performing ring closure with formamide to obtain a target mother ring; performing fluorination on carbonyl by using DAST; introducing difluorine into an active group so as to improve the bioactivity of the compound; performing process optimization on the basis of the compound; and performing the similar steps by using 2-(3-chlorphenyl)-2,2-difluoroacetic acid as a raw material to obtain the target product.
Owner:CGENETECH (SUZHOU CHINA) CO LTD
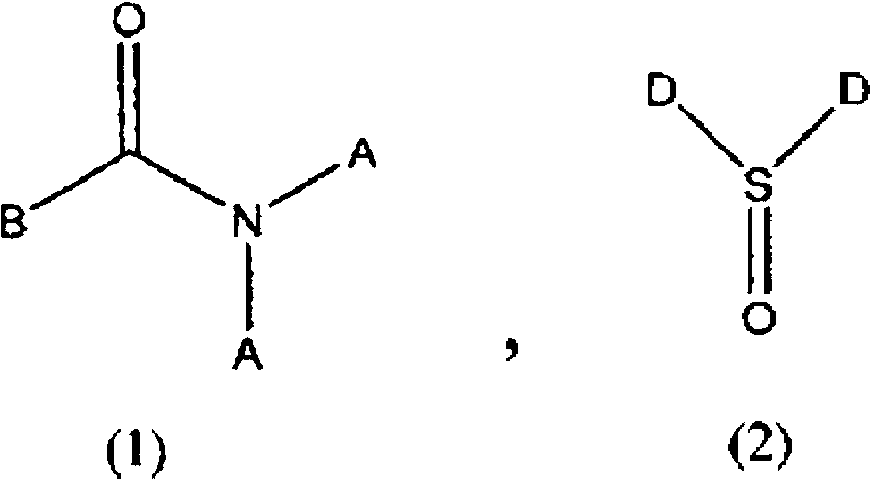
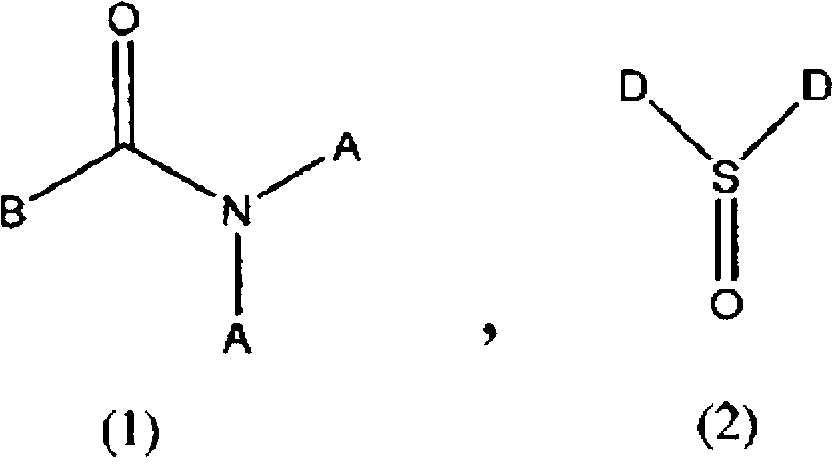
Method for producing difluoroacetic acid ester
InactiveCN102770405AEfficient recyclingEasy yieldPreparation from carboxylic acid halidesOrganic compound preparationHydrogen fluorideScavenger
Disclosed is a method for producing a difluoroacetic acid ester which involves at least: a reaction step in which a reaction solution is obtained by bring an alcohol represented by ROH into contact with a difluoroacetic acid fluoride (CHF2COF) in the presence of a hydrogen fluoride scavenger comprising an amide compound represented by formula (1) or a sulfone compound represented by formula (2); and a distillation step (A) in which a difluoroacetic acid ester represented by CHF2COOR is obtained as a distillate from the reaction solution obtained in the reaction step. As a consequence, said production method does not cause a drop in product yield attributable to hydrogen fluoride, which is a by-product of the mechanism of esterification reaction using difluoroacetic acid fluoride.
Owner:CENT GLASS CO LTD
Preparation method of difluoroacetic acid
ActiveCN109534987AReduce consumptionHigh catalytic efficiencyOrganic compound preparationCarboxylic compound preparationAcetic acidDifluoroacetic acid
The invention relates to a preparation method of difluoroacetic acid, and belongs to the technical field of fluorine chemical engineering. According to the preparation method of the difluoroacetic acid, KMnO4 / C solid catalysts are added into a fixed bed reaction kettle; nitrogen gas is introduced to perform replacement on the air in the reaction kettle; the temperature is raised to a fixed temperature; then, hydrogen peroxide and 2-chlorine-1,1-difluoroethane are introduced by a voltage stabilizing pump to perform contact reaction; after a product is separated, the product of difluoroacetic acid is finally obtained. The preparation method of the difluoroacetic acid has the advantages that the process is simple; the flow process is short; the technical flow process is simplified; the energyconsumption is greatly reduced; the method is suitable for industrialized production; the used raw materials are all common reagents, the resources of the materials are wide, and the price is low; the reaction process can be easily controlled; the obtained difluoroacetic acid product has high yield and has wide application and popularization values.
Owner:SHANDONG HUAAN NEW MATERIAL
Preparation method of 1-difluoroalkyl isoquinoline
ActiveCN106543081AEasy to separateMild reaction conditionsOrganic chemistryInorganic saltsIsoquinoline
The invention relates to the field of organic synthesis, in particular to a preparation method of 1-difluoroalkyl isoquinoline. The method comprises the following steps: taking alkenyl isocyanide and a difluoroacetic acid derivative as raw materials, taking palladium chloride as a catalyst, taking organic phosphine as a ligand and taking inorganic salt as alkali in a polar solvent under protection of inert gas; fully stirring at a certain temperature; after reaction reaches an end point, separating and purifying to obtain the 1-difluoroalkyl isoquinoline. The method not only is gentle in reaction condition, high in operability, low in cost, high in safety and environment-friendly, but also is high in reaction conversion percent, high in yield and short in technological process; the reaction scale is easy to expand; and therefore, the preparation method of the 1-difluoroalkyl isoquinoline has the advantage of suitability for industrial production.
Owner:SICHUAN UNIVERSITY OF SCIENCE AND ENGINEERING
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com