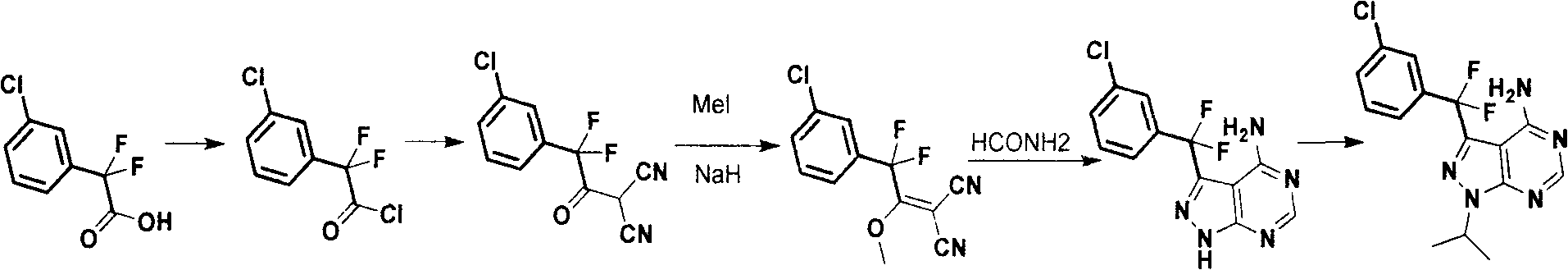
Synthesis process for anti-tumor new medicine 3-{(3-chlorphenyl)difluoromethyl-1-isopropyl-monohydro-pyrazolo(3,4-D)pyrimidine-4-amine compound
A difluoromethyl and chlorophenyl technology, applied in the field of medicine, can solve problems such as unsatisfactory treatment, unresponsiveness or drug resistance of cancer patients, and treatment failure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach
[0023] Preparation of 2-(3-chlorophenyl)-2,2-difluoroacetyl chloride:
[0024] 206.6 g (1 mole) of starting material 2-(3-chlorophenyl)-2,2-difluoroacetic acid was dissolved in 2 liters of dichloromethane, and 254 g (2 moles) of oxalyl chloride was added dropwise after adding 5 ml of DMF Under ice-water cooling, the solution was raised to room temperature and stirred for 3 hours, and spin-dried to obtain 225 g of acid chloride (yield 100%), which was directly used in the next step without purification.
[0025] Docking with acrylonitrile:
[0026] Dissolve 66 grams (1 mole) of malononitrile in 680 milliliters of DMF, cool to 0°C, add 44 grams (1.1 moles) of sodium hydrogen (60% purity) in batches, react at room temperature for 1 hour after the addition, and cool in an ice-salt bath Add dropwise the acid chloride obtained in the step, control the temperature below 5 degrees, and react overnight at room temperature after completion. TLC detects that the reaction is complete. (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com