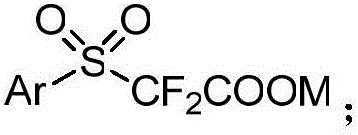
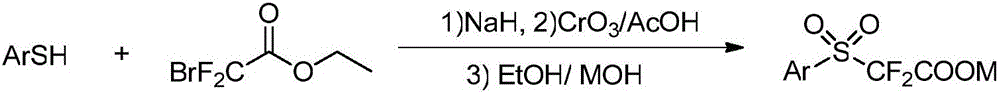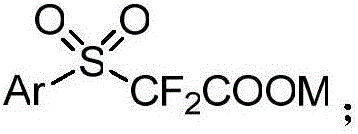Aryl sulfuryl difluoroacetic salt compound, as well as preparation method and application thereof
A technology for arylsulfone-based difluoroacetate and compound, which is applied in the field of organic synthesis, can solve the problems of few reports of -difluoromethyl alcohol compounds, harsh reaction conditions, difficult preparation of reaction reagents, etc., and achieves product yield. The effect of high rate, high universality and few side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] 1) At room temperature and normal pressure, put 2.25g (55mmol) NaH (60%) into a two-necked flask, and add 40ml DMSO. Under reflux of condensed water, 5.50 g (50 mmol) of thiophenol was slowly added dropwise, and reacted at room temperature for 1 h. Under the reflux of condensed water, 12.20 g (60 mmol) ethyl bromodifluoroacetate was slowly dropped into the mixture, and reacted at room temperature for 16 h. Saturated with NH in 50ml 4 Quench the reaction with Cl solution, extract with dichloromethane, combine the organic phases, wash with water and saturated NaCl aqueous solution successively, dry over anhydrous sodium sulfate, concentrate under reduced pressure, and separate by column chromatography to obtain the product 10.26g, yield 88%.
[0026] 2) Under normal pressure, 10.26g (40mmol) of the above-mentioned 17.60g (176mmol) CrO 3 , 50ml of acetic acid was put into the two-neck flask in turn, under the circulation of condensed water, the reaction was stirred a...
Embodiment 2
[0029]1) As described in Example 1, add 7.00 g (50 mmol) of p-methoxythiophenol, and add p-methoxythiophenol, sodium hydride, and ethyl difluorobromoacetate at a ratio of 1.0:1.1:1.2 at room temperature After reacting for 16 hours, we get 12.06g, yield 92%.
[0030] 2) As shown in Example 1, add The ratio of chromium trioxide was 1:4, the reaction was stirred at 60°C, and the reaction was monitored by TLC. After the disappearance of the raw materials, the reaction is stopped to obtain 11.91 g, yield 88%.
[0031] 3) As shown in Example 1, add The ratio of KOH is 1:1, the reaction is at room temperature, and the reaction is monitored by TLC. After the disappearance of the raw materials, the reaction is stopped to obtain 11.20 g, yield 91%.
Embodiment 3
[0033] 1) As described in Example 1, add 6.40 g (50 mmol) of p-fluorothiophenol, add p-fluorothiophenol, sodium hydride, and ethyl difluorobromoacetate at a ratio of 1.0:1.1:1.2, and react at 40°C for 16 hours, get 7.04g, yield 56%.
[0034] 2) As shown in Example 1, add The ratio of chromium trioxide was 1:4, the reaction was stirred at 60°C, and the reaction was monitored by TLC. After the disappearance of the raw materials, the reaction is stopped to obtain 6.52g, yield 82%.
[0035] 3) As shown in Example 1, add The ratio of NaOH was 1:1, the reaction was at room temperature, and the reaction was monitored by TLC. After the disappearance of the raw materials, the reaction is stopped to obtain 5.49g, yield 86%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com