Process for preparing 4.4-difluoro-2-amino butyric acid with optical activity and ester thereof
A technology of difluorocrotonate and optical activity, which is applied in the field of ester preparation, to achieve the effects of reasonable selection of reaction process, reduction of synthesis cost, and assurance of reproducibility and stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] 1. Synthesis of (S)-N-tert-butoxycarbonyl-4,4-difluoro-2-amino-butyric acid
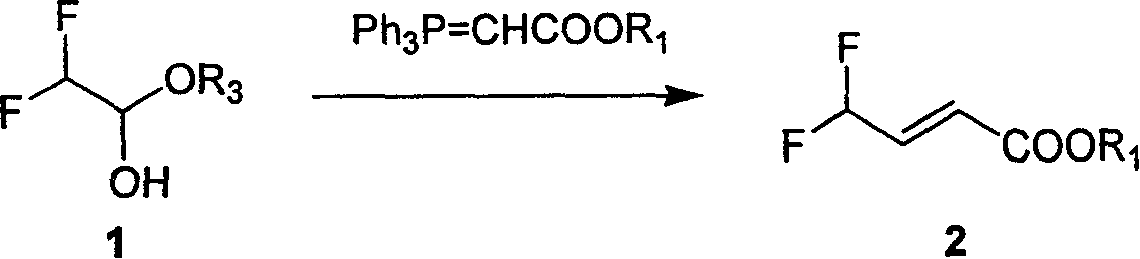
[0037] Step 1: Synthesis of 1-ethoxy-2,2-difluoroethanol
[0038] Ethyl difluoroacetate (300 mL, 3 mol) was dissolved in anhydrous THF (600 mL), cooled to -78°C under nitrogen protection, and a suspension of LAH (29 g, 0.75 mol) in anhydrous THF (800 mL) was added dropwise. After the dropwise addition was completed, stirring was continued at -78°C for 3 h, and 98% ethanol (75 mL) was added. Then the reaction solution was naturally warmed to 25°C, poured into ice water, and concentrated sulfuric acid (230 mL) was added with stirring. The reaction liquid was extracted with ether (2000 mL*4), the organic phases were combined, washed with saturated brine, dried over anhydrous magnesium sulfate, filtered to remove the desiccant, and the filtrate was spin-dried under reduced pressure (keep the temperature of the water bath below 30°C). The residue was distilled under reduced pressure (vacuum degre...
Embodiment 2
[0057] 1. Synthesis of (S)-N-tert-butoxycarbonyl-4,4-difluoro-2-amino-butyric acid
[0058] The second step: the synthesis of ethyl 4,4-difluoro-2-butenoate
[0059] Will 1-ethoxy-2,2-difluoroethanol (147g, 1.167mol), ethoxycarbonylmethenyltriphenylphosphine (487g, 1.400mol), p-toluenesulfonic acid (24g, 0.140mol) and anhydrous toluene (2.5L) were heated to 120°C and refluxed overnight. The solvent was distilled off under reduced pressure, diethyl ether (500 mL) was added to the residue, filtered, and the filter layer was washed with diethyl ether (200 mL). The filtrates were combined, the solvent was distilled off under reduced pressure, the residue was distilled under reduced pressure (0.95Mpa), and the fraction above 50°C was collected to obtain a colorless transparent liquid, which was 4,4-Difluoro-2-butenoic acid ethyl ester (136 g, 0.858 mol, yield: 78%). 1 H NMR (400MHz, CDCl 3 ): δ6.675(m, J=60.8Hz, 1H), δ6.200(m, J=120Hz 1H), δ4.209(m, J=28.0Hz, 2H), δ3.891(m, J...
Embodiment 3
[0061] The second step: the synthesis of ethyl 4,4-difluoro-2-butenoate
[0062] Will 1-ethoxy-2,2-difluoroethanol (147g, 1.167mol), ethoxycarbonylmethenyltriphenylphosphine (487g, 1.400mol), acetic acid (8.4g, 0.140mol) and anhydrous tetrahydrofuran (2.5L) were stirred overnight at 25°C. The solvent was distilled off under reduced pressure, diethyl ether (500 mL) was added to the residue, filtered, and the filter layer was washed with diethyl ether (200 mL). The filtrates were combined, the solvent was distilled off under reduced pressure, the residue was distilled under reduced pressure (0.95Mpa), and the fraction above 50°C was collected to obtain a colorless transparent liquid, which was 4,4-Difluoro-2-butenoic acid ethyl ester (106.8 g, 0.712 mol, yield: 61%).
[0063] 1 H NMR (400MHz, CDCl 3 ): δ6.675(m, J=60.8Hz, 1H), δ6.200(m, J=120Hz1H), δ4.209(m, J=28.0Hz, 2H), δ3.891(m, J=30.4 Hz, 1H); δ3.643(m, J=67.4Hz, 1H); δ1.296(t, J=69.6Hz, 1H), Ms(M + +1, 151).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com