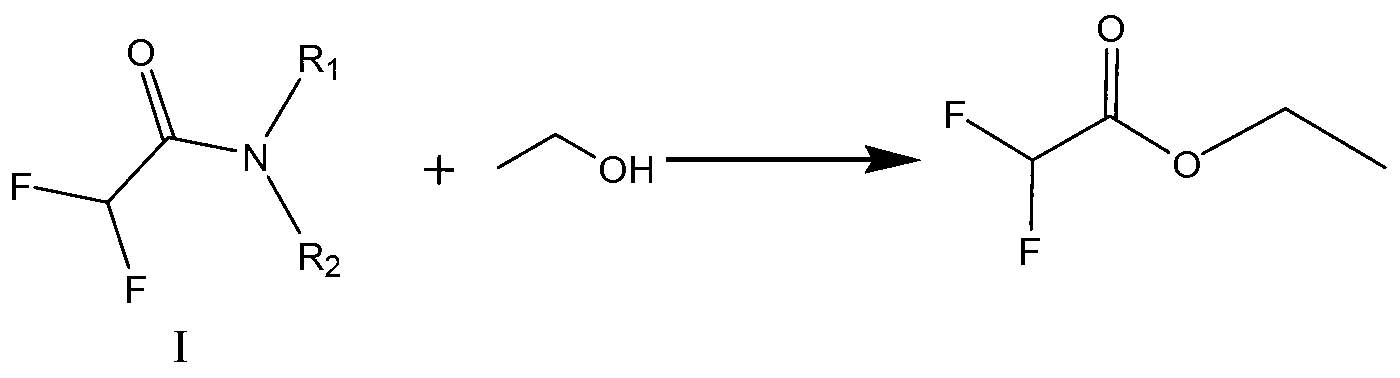
Preparation method of ethyl difluoroacetate and intermediate thereof
A technology of ethyl difluoroacetate and ethanol, which is applied in the field of preparation of ethyl difluoroacetate and its intermediates, can solve the problems of large amount of three wastes, high equipment requirements, and high production costs, and achieve the purpose of suppressing the production of ether and water, The effect of shortening reaction time and reducing product loss
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0081] Step 1. Add 73.2g (1mol) of methylisopropylamine and 400g of toluene to a 1000ml dry flask, add 147.4g (1mol) of dichloroacetyl chloride dropwise at 20-30°C, and keep warm for 0.5-1h after the addition. Add 147.4 g of water to wash the layers, and the organic layer is concentrated under reduced pressure to obtain 180.3 g (0.98 mol) of N-methyl-N-isopropyl-2,2-dichloroacetamide. The yield is 98%, and the purity is >99.0%.
[0082] Step 2. Add 132.5 (2.28mol) KF, catalyst p-tert-butyl[6]calixarene 8g, solvent DMF692g, 180.3g (0.98mol) N-methyl-N-isopropyl-2,2 to a 1000ml flask -Dichloroacetamide was kept at 90-100°C for 4-5h, filtered, concentrated by distillation to obtain 140.8g (0.931mol) N-methyl-N-isopropyl-2,2-difluoroacetamide, yield 95% ; Purity 99.5%.
[0083] Step 3. Add 184.2g (1.88mol) of concentrated sulfuric acid to a 500ml flask, and add 140.8g (0.931mol) of N-methyl-N-isopropyl-2,2-difluoroacetamide dropwise at a temperature of 95°C to 100°C And 45.2g (...
Embodiment 2
[0085] Step 1. Add 146.4g (2mol) of methylisopropylamine and 400g of toluene to a 1000ml dry flask, add 147.4g (1mol) of dichloroacetyl chloride dropwise at 20-30°C, and keep warm for 0.5-1h after the addition. Add 147.4 g of water to wash the layers, and the organic layer is concentrated under reduced pressure to obtain 181.8 g (0.988 mol) of N-methyl-N-isopropyl-2,2-dichloroacetamide. The yield is 99%, and the purity is >99.0%.
[0086] Step 2. Add 132.5 (2.28mol) KF, catalyst p-tert-butyl[6]calixarene 8g, solvent DMF692g, 181.8g (0.988mol) N-methyl-N-isopropyl-2,2 into a 1000ml flask - Dichloroacetamide was kept at 90-100°C for 4-5h, filtered, concentrated by distillation to obtain 141.8g (0.938mol) N-methyl-N-isopropyl-2,2-difluoroacetamide, yield 95% ; Purity 99.5%.
[0087]Step 3. Add 184.2g (1.88mol) of concentrated sulfuric acid to a 500ml flask, and add 141.8g (0.938mol) of N-methyl-N-isopropyl-2,2-difluoroacetamide dropwise at a temperature of 95°C to 100°C And 45...
Embodiment 3
[0089] Step 1. Add 182.8g (2.5mol) of methylisopropylamine and 400g of toluene into a 1000ml dry flask, add 147.4g (1mol) of dichloroacetyl chloride dropwise at 20-30°C, and keep warm for 0.5-1h after the addition. Add 147.4 g of water to wash the layers, and the organic layer is concentrated under reduced pressure to obtain 181.8 g (0.988 mol) of N-methyl-N-isopropyl-2,2-dichloroacetamide. The yield is 99%, and the purity is >99.0%.
[0090] Step 2. Add 278.2 (2.96mol) KF, 8g catalyst p-tert-butyl[6]calixarene, 692g solvent DMF, and 181.8g (0.988mol) N-methyl-N-isopropyl-2,2 to a 1000ml flask -Dichloroacetamide was kept at 90-100°C for 4-5h, filtered, concentrated by distillation to obtain 142.6g (0.943mol) N-methyl-N-isopropyl-2,2-difluoroacetamide, yield 95.4% ; Purity 99.5%.
[0091] Step 3. Add 184.2g (1.88mol) of concentrated sulfuric acid to a 500ml flask, and add 142.6g (0.943mol) of N-methyl-N-isopropyl-2,2-difluoroacetamide dropwise at 95°C to 100°C And 45.2g (0.9...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com