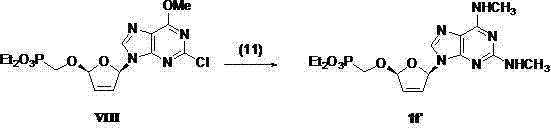Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
67 results about "Adenine nucleoside" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Purine base adenine attached to a ribose or deoxyribose.
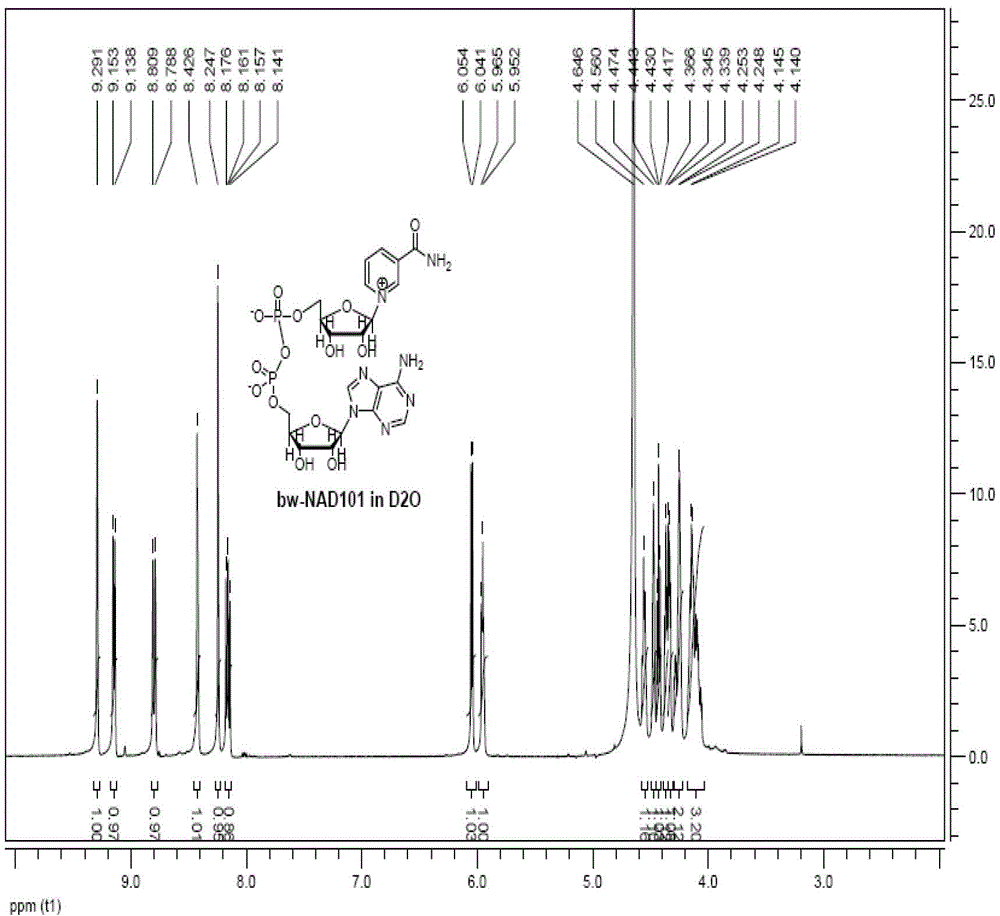
Preparation method of nicotinamide adenine dinucleotide
The invention relates to a preparation method of nicotinamide adenine dinucleotide, which comprises the following steps of: with 1,2,3,5-tetraacetyl-beta-D-ribofuranose as a starting raw material, preparing nicotinamide mononucleotide through sequentially a condensation reaction, an ammonolysis reaction and a phosphorylation reaction; and then mixing the prepared nicotinamide mononucleotide with adenosine triphosphate, generating an enzymic catalytic reaction in the presence of nicotinamide adenosine nucleotide transferase and pyrophosphatase to prepare the nicotinamide adenine dinucleotide. Compared with the prior art, the technical scheme provided by the invention has the advantage that the whole process is simplified and optimized by combination of a chemical method and an enzymic method.
Owner:SYNCOZYMES SHANGHAI
Organ preservative fluid and preparation method thereof
InactiveCN101496512AEffective preservationPrevent ischemia-reperfusion injuryDead animal preservationReperfusion injuryTetramethylpyrazine hydrochloride
The invention relates to a preservation solution for organs, tissues or cells of human bodies or animals. The organ preservation solution comprises sodium citrate, potassium citrate, magnesium sulfate, sodium dihydrogen phosphate, sodium hydroxide, adenosine, arginine, tryptophan, mannitol, tetramethylpyrazine hydrochloride and other components. The organ preservation solution can be used for cooling, lavaging and preserving the organs of the human bodies or the animals, can effectively preserve human in vitro kidneys for 48 hours and animal in vitro kidneys for 72 hours, can prevent ischemia-reperfusion injury, and has great value for clinical application.
Owner:YANGZIJIANG PHARMA GROUP SHANGHAI HAINI PHARMA
Purine derivatives as a3 adenosine receptor-selective agonists
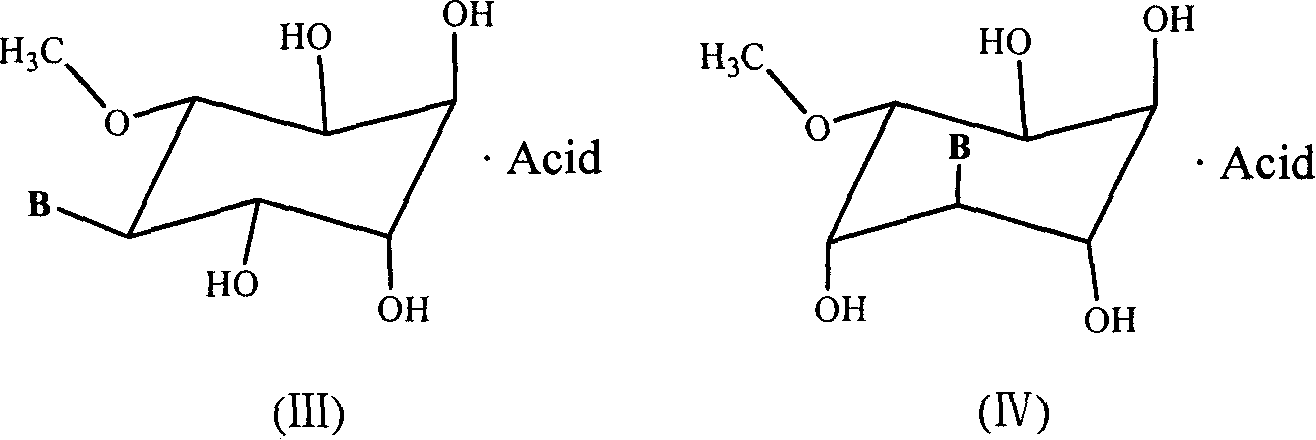
Disclosed are (N)-methanocarba adenine nucleosides, e.g., of formula (I) as highly potent A3 adenosine receptor agonists, pharmaceutical compositions comprising such nucleosides, and a method of use of these nucleosides, wherein R1-R6 are as defined in the specification. These nucleosides exhibit similar selectivities as agonists of the A3 versus the A1 receptor for both human and mouse adenosine receptors, and are contemplated for use in the treatment a number of diseases, for example, inflammation, cardiac ischemia, stroke, asthma, diabetes, and cardiac arrhythmias.
Owner:UNITED STATES OF AMERICA
A3 adenosine receptor agonists and antagonists
Disclosed are (N)-methanocarba adenine nucleosides of formulas (I)-(V), for example, of formula (V):as highly potent A3 adenosine receptor agonists, pharmaceutical compositions comprising such nucleosides, and a method of use of these nucleosides, wherein R1-R6 are as defined in the specification. These nucleosides exhibit similar selectivities as agonists of the A3 versus the A1 receptor for both human and mouse adenosine receptors, and are contemplated for use in the treatment a number of diseases, for example, inflammation, cardiac ischemia, stroke, asthma, diabetes, and cardiac arrhythmias.
Owner:US DEPT OF HEALTH & HUMAN SERVICES
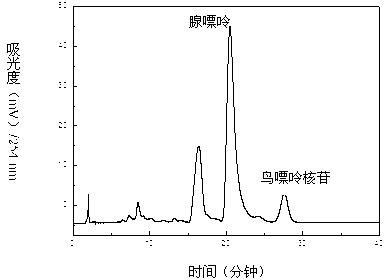
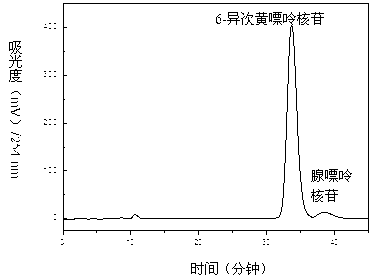
Method for separating and purifying four nucleoside chemical ingredients from trichosanthes bark
ActiveCN103304613ASimple compositionNot pollutedSugar derivativesChemical recyclingPharmacologyChinese herbology
The invention relates to a method for separating and purifying four nucleoside chemical ingredients (namely adenine, guanosine, 6-iso inosine and adenosine) from traditional Chinese medicine trichosanthes bark. According to the method, four high-purity monomeric compounds, namely adenine, guanosine, 6-iso inosine and adenosine, are obtained from the trichosanthes bark through the following steps of: (1) preparing a crude trichosanthes bark extract; (2) carrying out crude separation by using a macroporous adsorption resin column; and (3) carrying out separation and purification by using semi-preparative high-performance liquid chromatography: carrying out separation and purification on obtained samples by using the semi-preparative high-performance liquid chromatography, wherein the mobile phase is methanol-water. The method disclosed by the invention has the advantages that the process flow is environment-friendly, the damage to the environment is not serious, and the comprehensive cost is low.
Owner:SHANDONG UNIV OF TRADITIONAL CHINESE MEDICINE +2
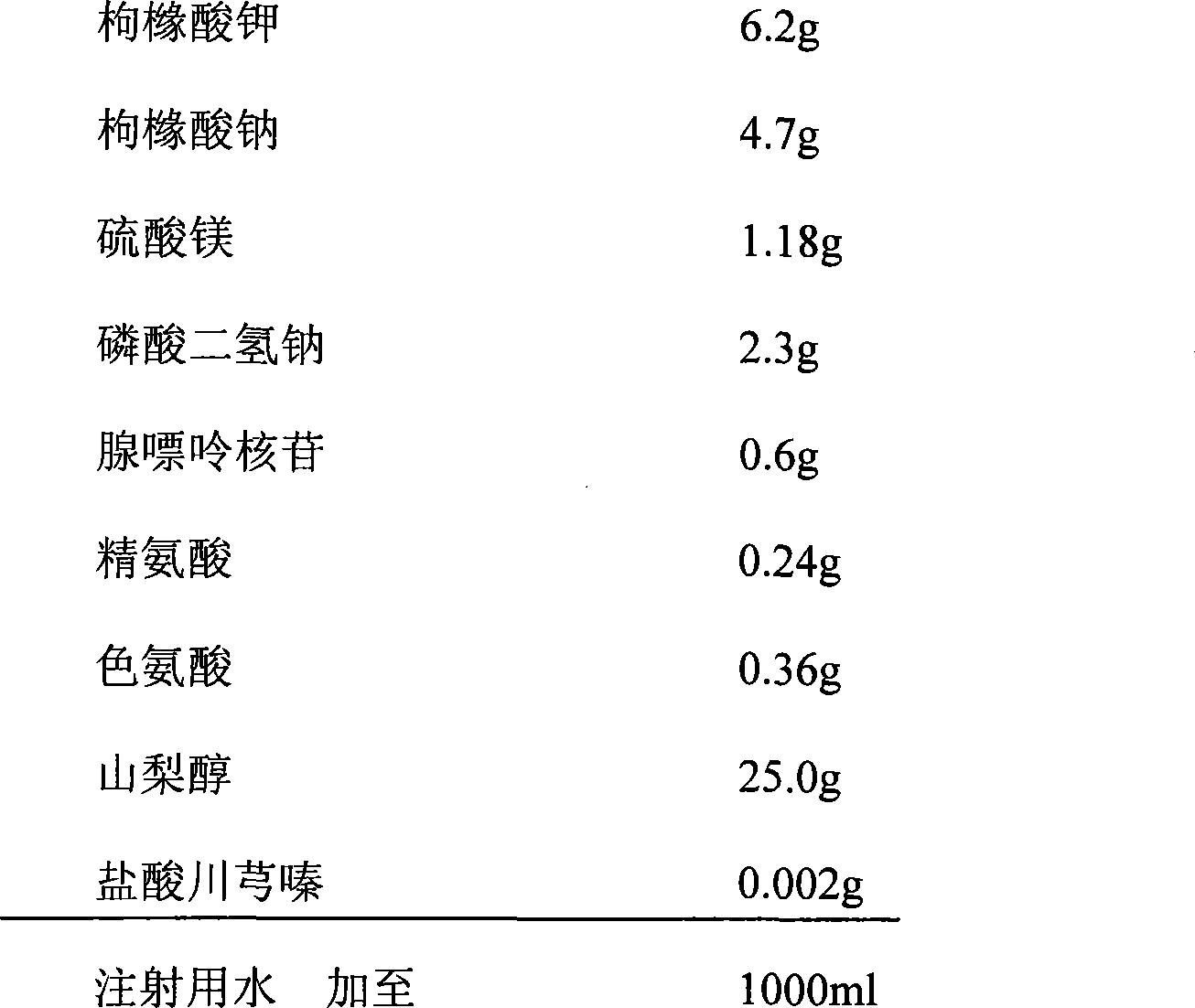
Kidney preservative fluid
InactiveCN1633848AAnti-lipid peroxidationMaintain hypertonic propertiesDead animal preservationAdditive ingredientArginine
The invention provides a kidney preservative fluid which comprises potassium citrate, sodium citrate, magnesium sulfate, disodium hydrogen phosphate, sodium dihydrogen phosphate, adenosine, L-arginine, ligustrazine and mannidex, which has the advantages of high penetrability, acidity stability, thus can prevent ischemia re-impregnating damage and conserve kidney as long as 72 hours.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Method for cultivating Tibetan lucidum
ActiveCN104488565AIncrease productionIncrease contentCultivating equipmentsMushroom cultivationEconomic benefitsIn vivo
The invention discloses a method for cultivating Tibetan lucidum. The method comprises the steps of strain isolation, fungi bag preparation, lucidum cultivation, bud pressing management, lucidum cultivation and management, and harvesting. According to the method provided by the invention, the contents of macromolecular polysaccharide, triterpenes, adenosine, alkaloid and various enzymes can be enhanced, the in vivo environment can be effectively adjusted, the immunologic function can be enhanced, the metabolism can be promoted, blood purification can be realized, circulation can be improved, body function can be conditioned, the manual cultivation can be realized at the first time, the cultivation period is short, the yield is high, the quality is good, and the social and economic benefits are high.
Owner:VEGETABLE RES INST OF TIBET ACADEMY OF AGRI & ANIMAL HUSBANDRY SCI
Purine derivatives as A3 and A1 adenosine receptor agonists
Disclosed are (N)-methanocarba adenine nucleosides of the formula:as highly potent A3 adenosine receptor agonists, pharmaceutical compositions comprising such nucleosides, and a method of use of these nucleosides, wherein R1-R6 are as defined in the specification. These nucleosides are contemplated for use in the treatment a number of diseases, for example, inflammation, cardiac ischemia, stroke, asthma, diabetes, and cardiac arrhythmias. The invention also provides compounds that are agonists of both A1 and A3 adenosine receptors for use in cardioprotection.
Owner:GOVERNMENT OF THE US REPRESENTED BY THE SEC
A3 adenosine receptor agonists and antagonists
Disclosed are (N)-methanocarba adenine nucleosides of formulas (I)-(V), for example, of formula (V):as highly potent A3 adenosine receptor agonists, pharmaceutical compositions comprising such nucleosides, and a method of use of these nucleosides, wherein R1-R6 are as defined in the specification. These nucleosides exhibit similar selectivities as agonists of the A3 versus the A1 receptor for both human and mouse adenosine receptors, and are contemplated for use in the treatment a number of diseases, for example, inflammation, cardiac ischemia, stroke, asthma, diabetes, and cardiac arrhythmias.
Owner:UNITED STATES OF AMERICA
Methanocarba adenosine derivatives, pharmaceutical compositions, and method of reducing intraocular pressure
Disclosed are (N)-methanocarba adenine nucleosides, e.g., of the formula (I): as A3 adenosine receptor agonists, pharmaceutical compositions comprising such nucleosides, and a method of use of these nucleosides, wherein A, a, R2, and R3 are as defined in the specification. These nucleosides are contemplated for use in the treatment a number of diseases, for example, inflammation, cardiac ischemia, stroke, asthma, diabetes, and cardiac arrhythmias. Also disclosed are conjugates comprising a dendrimer and one or more ligands, which are functionalized congeners of an agonist or antagonist of a receptor of the G-protein coupled receptor (GPCR) superfamily. Such conjugates are have the potential of being used as dual agonists, dual antagonists, or agonist / antagonist combinations.
Owner:UNITED STATES OF AMERICA
Water-soluble cationic biphenyl aromatic hydrocarbon as well as inclusion compound and preparation method thereof
ActiveCN108610243AMild reaction conditionsSimple and fast operationOrganic compound preparationPharmaceutical non-active ingredientsStructural formulaPi interaction
The invention relates to a water-soluble cationic biphenyl aromatic hydrocarbon as well as an inclusion compound and a preparation method thereof. The compound has a structural formula as shown in thedescription, wherein n is equal to 4-7. The inclusion compound is molecular aggregate formed by the steps: taking water-soluble cationic biphenyl [4] aromatic hydrocarbon as a macrocyclic host compound, taking an ATP molecule (Adenosine Triphosphate) as an individual guest molecule, enabling the ATP molecule to enter a cavity of the water-soluble cationic biphenyl [4] aromatic hydrocarbon, and forming the aggregate by virtue of electrostatic interaction and pi-pi interaction. A molar ratio of the water-soluble cationic biphenyl [4] aromatic hydrocarbon to the ATP molecule is 1 to 1. A bindingconstant measured by a thermostatic calorimetric titration experiment (ITC) experiment is (1.05+ / -0.07)*10<4>M<-1>. The method has the advantages of being simple in operation and mild in reaction conditions, improves the biological stability of the ATP, and provides a new thought for reducing multidrug resistance (MDR) produced by tumor cells to anticancer drugs.
Owner:SHANGHAI UNIV
Purine derivatives as A3 adenosine receptor-selective agonists
Disclosed are (N)-methanocarba adenine nucleosides, e.g., of formula (I) as highly potent A3 adenosine receptor agonists, pharmaceutical compositions comprising such nucleosides, and a method of use of these nucleosides, wherein R1-R6 are as defined in the specification. These nucleosides exhibit similar selectivities as agonists of the A3 versus the A1 receptor for both human and mouse adenosine receptors, and are contemplated for use in the treatment a number of diseases, for example, inflammation, cardiac ischemia, stroke, asthma, diabetes, and cardiac arrhythmias.
Owner:UNITED STATES OF AMERICA
Method for detecting adenosine triphosphate by paper-based dual-mode electrochemical sensor
InactiveCN110441357AHigh-precision detectionReduce experiment costMaterial electrochemical variablesDual modeMulti method
The invention relates to a method for detecting adenosine triphosphate by a paper-based dual-mode electrochemical sensor and discloses a method for detecting adenosine triphosphate in a paper-based dual-mode manner. A hydrophobic region and a hydrophilic region are fabricated on paper by a wax printing technology, an electrode and a wire are printed by a silk-screen printing technology, and folding equipment is formed by a cutting technology. Different regions of a paper chip are functionalized by various types of methods, an instantaneous current is detected by a universal meter by specific identification effects of the adenosine triphosphate and an aptamer, specificity catalysis of glucose oxidase on glucose and an energy storage effect of a supercapacitor, so that the sensitivity is improved, and ultra-sensitive electrochemical detection of a to-be-detected object can be achieved by an electrochemical working station.
Owner:UNIV OF JINAN
A large-scale serum-free culture method of rhil-12 engineered cells
The invention relates to a large-scale serum-free culture method for rhIL-12 engineering cells, which comprises the following step of: inoculating rhIL-12 engineering cells in a logarithmic phase into a serum-free and protein-free medium for culture, wherein the medium is a CD CHO liquid medium comprising sodium pyruvate at the final concentration of 0.8 to 1.2mM, hypoxanthine at the final concentration of 0.075 to 0.125mM, thymidine at the final concentration of 0.012 to 0.020mM, adenosine at the final concentration of 0.5 to 0.9mg / L, guanosine at the final concentration of 0.5 to 0.9mg / L, cytidine at the final concentration of 0.5 to 0.9mg / L, uridine at the final concentration of 0.5 to 0.9mg / L, L-glutamine at the final concentration of 0.4 to 0.8mg / L, L-asparagine at the final concentration of 0.4 to 0.8mg / L, L-proline at the final concentration of 1.5 to 2.0mg / L and non-essential amino acid at the final concentration of 0.08 to 0.125mM. The invention also provides a medium used in the method. By the medium and the culture method, a high-yield and high-activity recombinant human interleukin-12 can be obtained.
Owner:UNIV OF SCI & TECH OF CHINA
Carbon dioxide kit employing enzymatic cycling assay
The invention discloses a carbon dioxide kit employing enzymatic cycling assay. The kit is composed of a NADH analogue cyclic regeneration system and a carbon dioxide test reagent, wherein the NADH analogue cyclic regeneration system consists of 6-phosphogluconate dehydrogenase, hexokinase, glucose and adenosine triphosphate disodium. The carbon dioxide kit is simple to prepare and low in cost, and the NADH analogue cyclic regeneration system in the kit can prolong the storage time of the kit and effectively improves the stability, accuracy and precision of testing.
Owner:ZHONGSHAN CHUANGYI BIOCHEM ENG
Quality control method for ribonucleic acid II for injection
ActiveCN102818867AHigh technology contentImprove securityComponent separationInternational marketHydrolysis
The invention discloses a quality control method for ribonucleic acid II for injection. The method comprises the following steps that the nucleic acid enzyme hydrolysis solution of a substance to be measured is subjected to high-performance liquid chromatogram analysis, an adopted chromatographic column is an Agilent ZORBAX SB-AQC18 chromatographic column, and a flowing phase is a mixture of a formic acid solution and an acetonitrile solution; after the analysis, if the substance to be measured is determined to contain five substances as follows: cytidylate, uridine monophosphate, guanine nucleotide, guanosine and adenosine, the substance to be measured is the ribonucleic acid II for injection or is the ribonucleic acid II for injection as a candidate; and if not, the substance to be measured is not the ribonucleic acid II for injection or is not the ribonucleic acid II for injection as the candidate. A high-performance liquid chromatographic technique is utilized, and the strong-specificity quality control method for the ribonucleic acid II for injection is established. The method has important meanings on increasing the technological content of the medicine, increasing the safety and effectiveness, reducing the cost, enlarging the production scale, increasing the market occupancy, and going forward to the international market.
Owner:JILIN AODONG PHARMACEUTICAL INDUSTRY GROUP YANJI CO LTD
Adenosine deaminase detection kit
InactiveCN105586387AGood colorRapid responseMicrobiological testing/measurementBiological material analysisPeroxidaseBovine serum albumin
The invention relates to an adenosine deaminase detection kit. A vessel is contained in the detection kit and filled with liquid for detection, the liquid for detection is prepared from 50-100 mmol / L of glycine buffer solution, 50-80 mmol / L of sodium benzoate, 12 / 18 u / L of purine nucleoside phosphorylase, 30-40 u / L of xanthine oxidase, 200-300 u / L of peroxidase, 30-100 g / L of glyceraldehyde, 0.5 g / L of sodium azide, 10-200 mmol / L of disodium hydrogen phosphate, 100-300 mmol / L of KCl, 40-100 mmol / L of mannitol, 0.1-0.3 ml / L of preservative, 100-150 mmol / L of adenosine, 2-4 mmol / L of 4-aminoantipyrine, 10-15 mmol / L of color developing agent, 20-40 mmol / L of adenine nucleoside and 3-5 g / L of bovine serum albumin. The detection kit has the advantages of being high in determination precision, high in antijamming capacity, suitable for automated rapid determination and capable of creating favorable conditions for routine development of ADA detection application in clinic.
Owner:VISION BIOLOGICAL TECH HEFEI CO LTD
Gene therapy for cancer using small interfering RNA specific to ant2 and a method to overcome tolerance to antitumor agent
InactiveUS20090202623A1Prevent proliferationReduce doseOrganic active ingredientsSugar derivativesTolerabilityAnticarcinogen
The present invention relates to a small interfering RNA (siRNA) suppressing the expression of adenine nucleotide trnaslocator 2 (ANT2) gene and an anticancer agent containing the same. Particularly, the invention relates to ANT2 siRNA comprising a sense sequence selected from the nucleotide sequences of ANT2 mRNA, a hairpin loop sequence and an antisense sequence binding complementarily to the said sense sequence and an anticancer agent containing the same. ANT2 siRNA of the present invention inhibits the expression of ANT2 gene, suggesting that it inhibits the growth of cancer cells exhibiting high level of ANT2. Therefore, ANT2 siRNA of the invention can be effectively used for gene therapy for cancer treatment and further prevents the anticancer effect from decreasing by anticancer drug resistance of cancer cells.
Owner:BIOINFRA
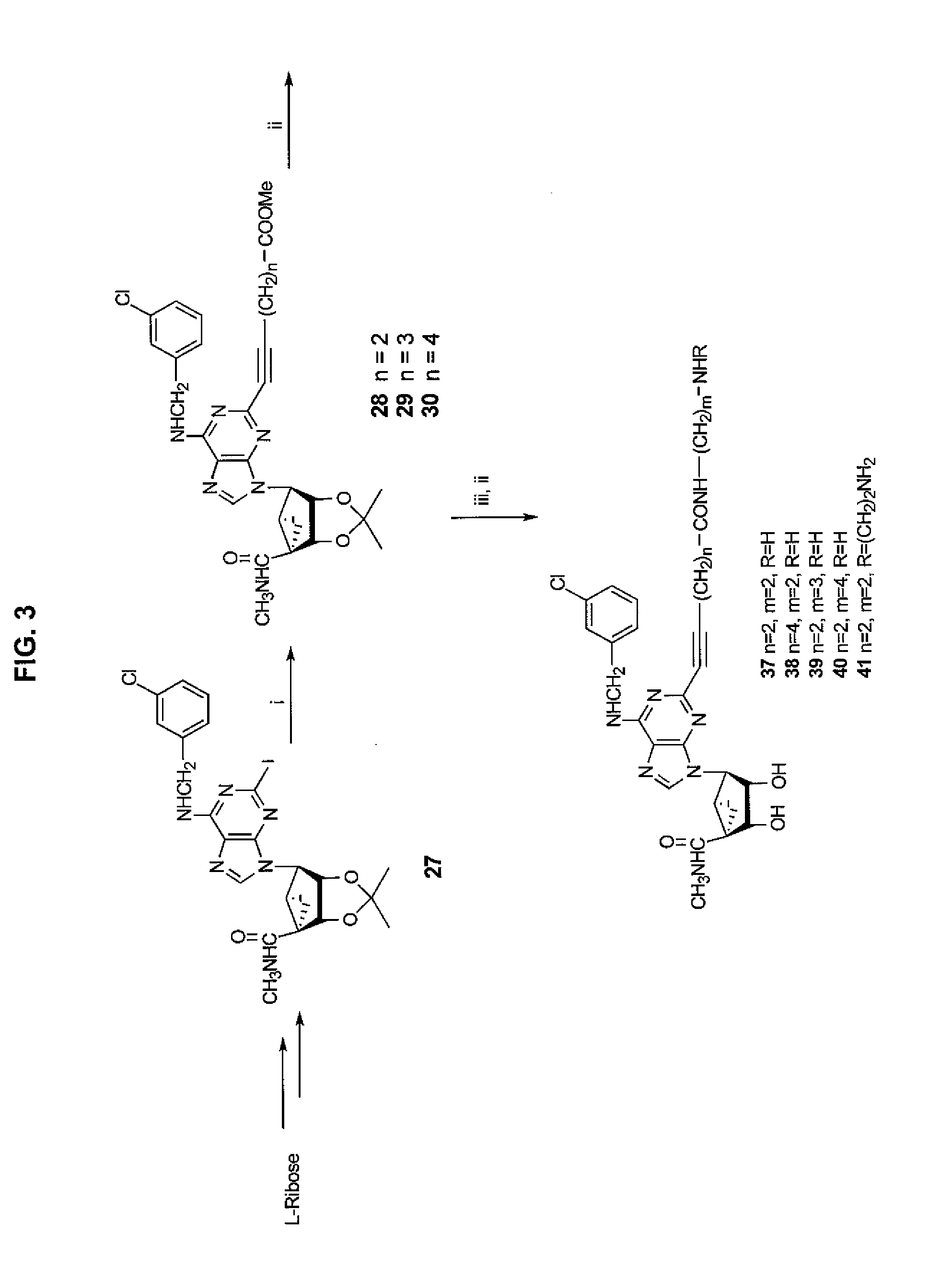
A group of six-carbocycle nucleoside analogue, its synthesis method and antiviral application
InactiveCN1803819ASaccharide with heterocyclic radicalsOrganic active ingredientsSodium bicarbonate5-fluorocytidine
The disclosed six-membered carbocyclic nucleoside analogues comprise: adenosine analogue, guanosine analogue, carnine analogue, mercaptopurine riboside analogue, cytidine analogue, 5-fluorocytidine analogue, uridine analogue, 5-fluorouridine analogue, and thymidine analogue as well as their acceptable salts formed by equimolar acid in pharmacy. Wherein, the opposite five-step synthesis method using the pinitol, acetone, methane sulfonyl chloride, p-toluenesulfonyl chloride, benzene sulfochloride, and nucleoside base as materials; the pyridine, water, glacial acetic acid, absolute methanol, DMSO, N, N-DMF as the solvent; the p-toluenesulfonic acid, 2, 2-dimethoxylpropane, anhydrous NaSO4, NaHCO3, triethylamine, and anhydrous K2CO3 as the catalyst. This invention restrains specially the replication of HIV and herpesvirus.
Owner:SHANDONG UNIV
Adenosine deaminase (ADA) detection reagent kit and preparation method thereof
The invention discloses a detection reagent kit for detecting human ADA. A reagent kit body contains a glycine buffer solution, a color-developing agent prepared with 3-methyl-N,N-dipropyl sodium sulfonate aniline, purine nucleoside phosphorylase, xanthine oxidase, peroxidase, adenosine and a 4-aminoantipyrine solution. A sample and a reagent are mixed in a certain volume proportion and subjected to a series of reactions, then reactants are placed under a semi-automatic / full-automatic biochemical analyzer, and the change rate of the absorbency at a position of 550nm dominant wavelength is detected, so that the concentration of the ADA is measured and calculated. The reagent kit has the advantages of being accurate, stable and convenient.
Owner:乐普(北京)诊断技术股份有限公司
Method for hydrolyzing adenosine through cation exchange resin catalysis-separation coupled technology
ActiveCN109575028AReduce manufacturing costPromote crystallizationSugar derivativesOrganic chemistry methodsHydrogenNitrogen
The invention discloses a method for hydrolyzing adenosine through cation exchange resin catalysis-separation coupled technology and belongs to the technical field of biochemical engineering. According to the method for hydrolyzing the adenosine through the cation exchange resin catalysis-separation coupled technology, catalysis-absorption coupled interaction of cation exchange resin is applied tocutting off carbon-hydrogen bonds in the molecular structure of adenosine and then efficiently hydrolyzing the adenosine to obtain adenine and meanwhile purify mother liquor containing D-ribose, thereby facilitating crystallization of D-ribose. The method for hydrolyzing the adenosine through the cation exchange resin catalysis-separation coupled technology can effectively reduce the production cost of the adenine and the D-ribose and improve the production efficiency and is applicable to scale production.
Owner:XINXIANG MEDICAL UNIV
Method for detecting effective ingredient content of cordyceps capsules
InactiveCN102128866ASimple and fast operationSensitive and accurate test resultsMaterial electrochemical variablesCordycepsInfrared lamp
The invention discloses a method for detecting effective ingredient content of cordyceps capsules. A multi-wall nano tube / Au (MWNT) / Au modified electrode and a stripping voltammetry are used for detection. The preparation of the modified electrode comprises the following steps of: (1) performing ultrasonic purification on MWNT in concentrated hydrochloric acid for 7.5 hours, refluxing the purified MWNT for 8 hours at the temperature of 140 DEG C by adding concentrated nitric acid, performing secondary washing till neutrality, drying the washed product at the temperature of 100 DEG C, and grinding the dried product into powder, namely functional MWNT powder; and (2) ultrasonically dispersing the functional MWNT powder obtained in the step (1) into dimethyl formamide (DMF) to form black suspension, dripping the suspension on the surface of a gold electrode by using a trace sample injector, and drying the suspension under an infrared lamp to obtain the MWNT / Au modified electrode. The method for detecting the content of adenosine serving as the effective ingredient of the cordyceps capsules is economic and simple and convenient in operation, and the detection result is accurate and sensitive.
Owner:DALIAN UNIVERSITY
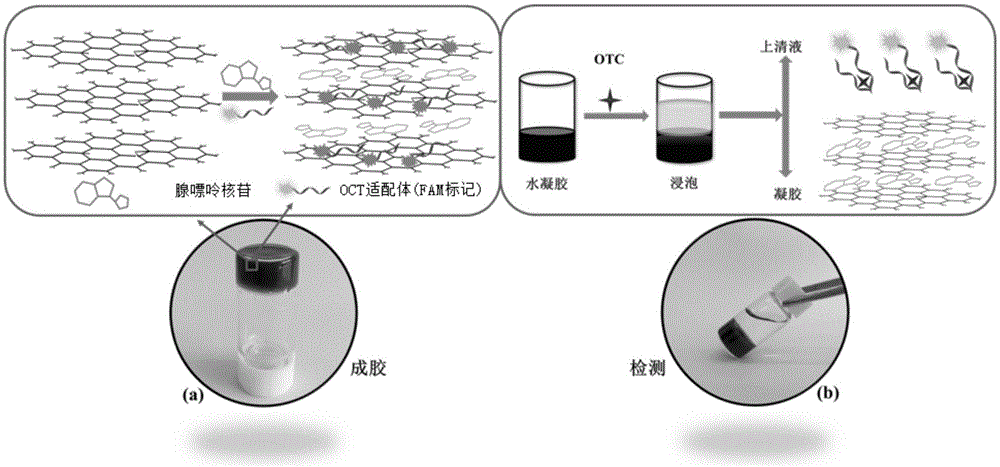
Oxytetracycline fluorescence detection method based on graphene-based compound hydrogel
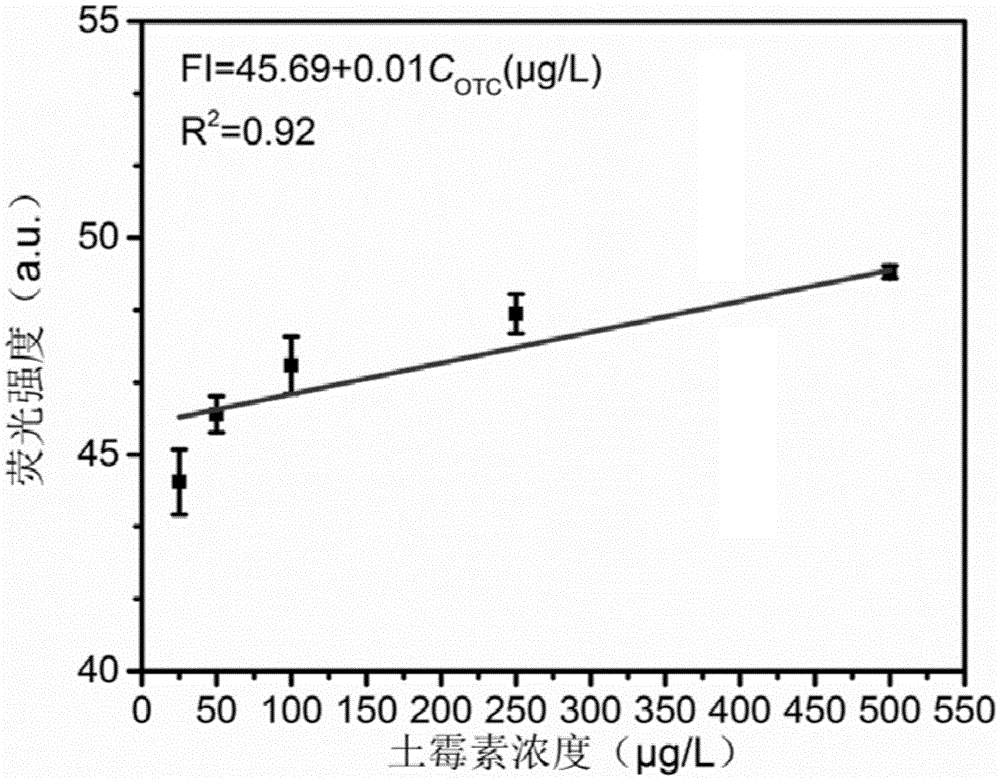
ActiveCN105572090AEasy to detectAchieve controllable detection rangeFluorescence/phosphorescenceWater basedAptamer
The invention belongs to the technical field of environmental monitoring, and relates to an oxytetracycline fluorescence detection method for antibiotics in water based on graphene-based compound hydrogel. Graphene dispersed liquid is adopted as a monomer, adenosine and an aptamer are adopted as a cross-linking agent together, graphene oxide lamellas originally dispersed in a solution are connected together, and a three-dimensional macrostructure is formed. Oxytetracycline aqueous solutions with different concentrations are added to a hydrogel system for a simple soaking process, supernate is taken for fluorescence intensity testing, and quantitative fluorescence detection of the concentration of oxytetracycline can be achieved. In the prepared hydrogel system with different proportions, the detection range of oxytetracycline can be controllably adjusted, and the method can be applied to different types of actual water for detection. Compared with a traditional nano material sensing detecting method, the method is fast in nano material preparation, simple, convenient to use, mild in preparation condition and high in environmental stability and practicality of the hydrogel material.
Owner:DALIAN UNIV OF TECH
Adenosine deaminase detection reagent
InactiveCN106893759AGood colorRapid responseMicrobiological testing/measurementPeroxidasePolyethylene glycol
The invention relates to an adenosine deaminase detection reagent. The adenosine deaminase detection reagent contains the following components: 6u / L-10u / L of purin-nukleosid-phosphorylase, 20mmol / L-40mmol / L of a glycine buffer solution, 20mmol / L-30mmol / L of mannitol, 20mmol / L-40mmol / L of sodium benzoate, 10u / L-20u / L of xanthine oxidase, 0.05g / L of disodium ethylenediamine tetraacetic acid, 10g / L-20g / L of glyceraldehydes, 0.3g / L of sodium azide, 20mmol / L-100mmol / L of disodium hydrogen phosphate, 50u / L-100u / L of peroxidase, 80mmol / L-90mmol / L of KCl, 2mmol / L of N-ethyl N-(2-hydroxy-3-sulfopropyl)-3-methylaniline sodium salt, 0.4ml / L-0.5ml / L of a preservative, 80mmol / L-95mmol / L of adenosine, 5mmol / L-8mmol / L of 4-amino antipyrine, 20mmol / L-30mmol / L of a developing agent, 50mmol / L-70mmol / L of adenine nucleoside, 6g / L-8g / L of bovine serum albumin and 0.3g / L of polyethylene glycol 6000. The adenosine deaminase detection reagent has high determination precision and strong disturbance resistance and is suitable for automatic rapid determination.
Owner:徐淼
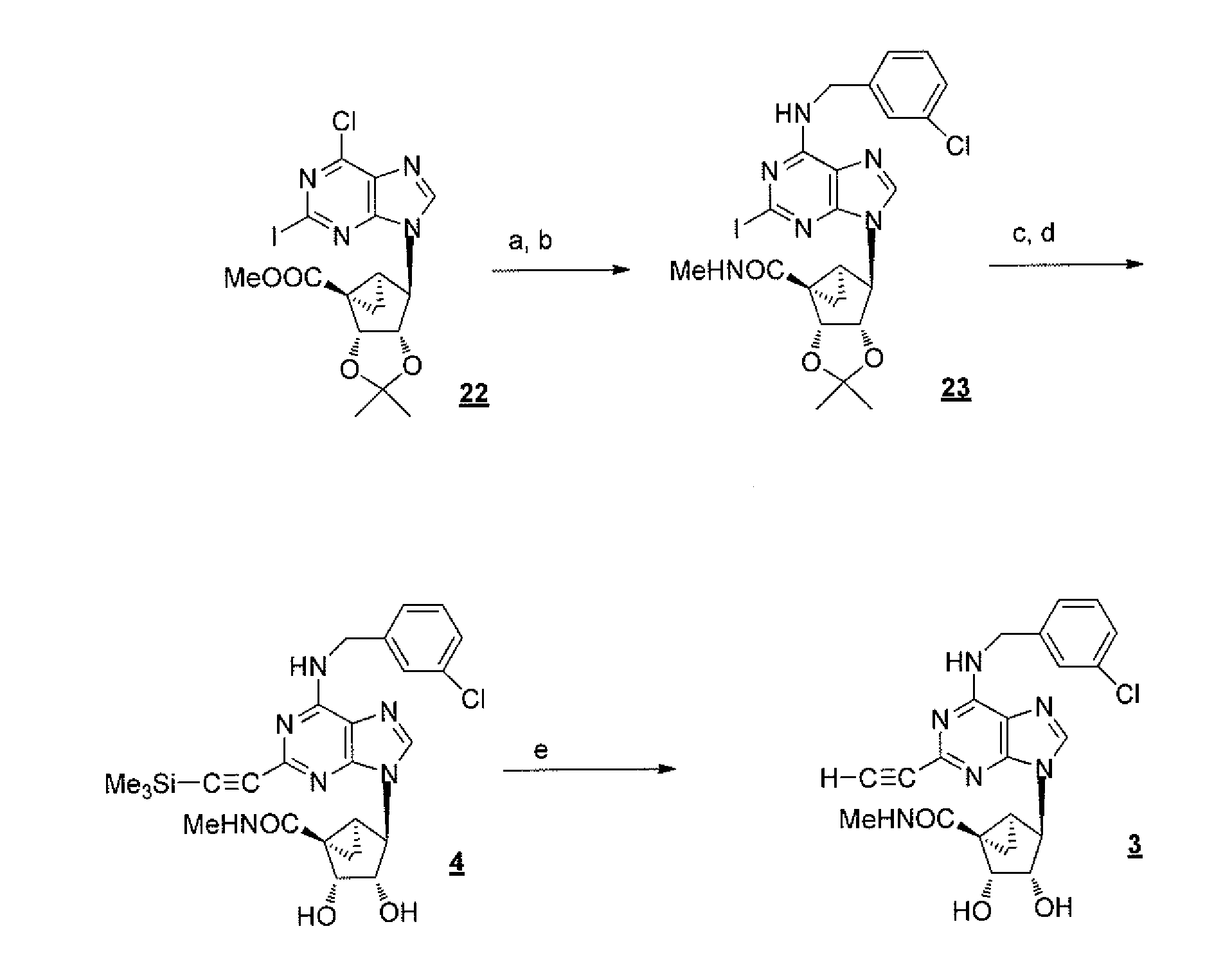
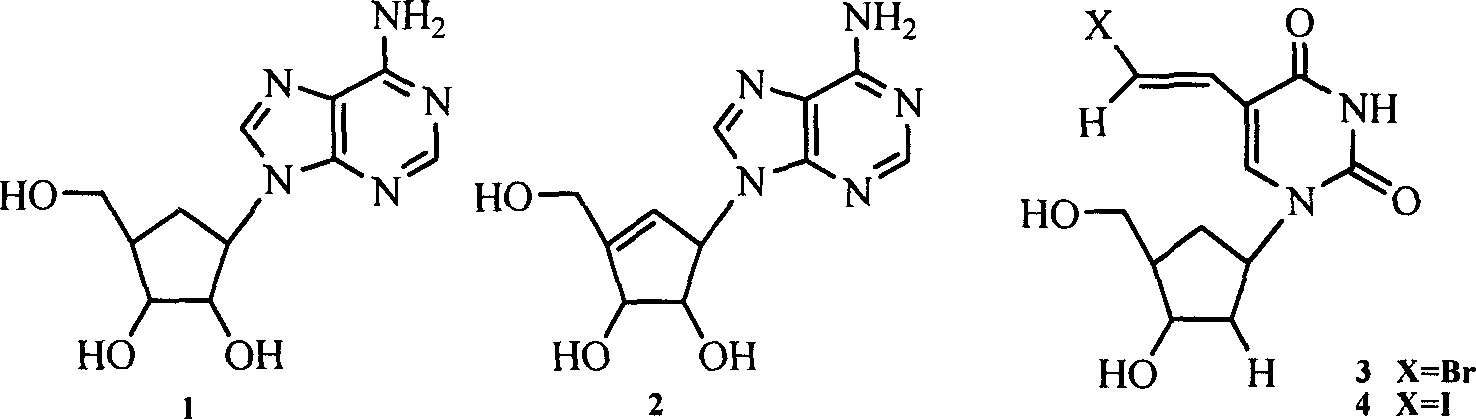
(2R,5R)-5-phosphoryl methoxy-2-(2-substituted adenine-9-yl)-2,5-dihydrofuran nucleoside analog as well as preparation method and application thereof
InactiveCN103788160AImprove stabilityExcellent resistance to nucleolytic enzyme decompositionOrganic active ingredientsSugar derivativesFuranPurine
The invention discloses a novel beta-furan phosphonate purine nucleoside analog, and in particular relates to a (2R,5R)-5-phosphoryl methoxy-2-(2-substituted adenine-9-yl)-2,5-dihydrofuran nucleoside analog as well as a preparation method and an application thereof, which belongs to the field of nucleoside chemicals and pharmaceutical chemistry. The analog has a structure as shown in the formula (1) in the specification, wherein R1 represents C1, NH2, OCH3, SCH3, NHCH3 or NHNH2; R2 represents NH2, OCH3 and NHCH3; R' represents H, Na or K. Chlorinated sugar with high activity is formed through chlorination of 3.5-di-O-p-methyl benzoyl-2-deoxidation-D-ribose glucoside, the chlorinated sugar is subsequently reacted with alkali, and a novel beta-D-dihydrofuran phosphonate adenine nucleoside analog is formed through selective oxidation, decarboxylation, addition, elimination, functional group conversion and ethyl removal. The analog has anti-virus activity and good development prospect.
Owner:ZHENGZHOU UNIV
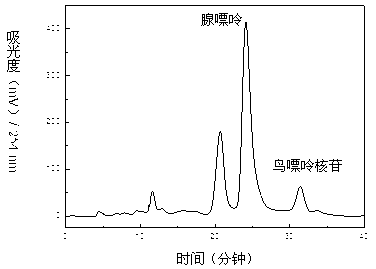
HPLC content determination method for adenosine in semen plantaginis
Adenosine, cannabiside B and ethyl glucoside are firstly separated and obtained from semen plantaginis, and on the above basis, the invention provides a method of determining the content of adenosine in semen plantaginis by utilizing a HPLC process. The chromatographic conditions of the method comprise that the mobile phase is acetonitrile and water according to the volume ratio of 5:95, the low velocity is 0.1 mL / min, the column temperature is 25 DEG C, the detection wavelength is 255 nm, and the sample size is 20 mu L. The method provides content determination index for completing quality standard of semen plantaginis, and also provides new scientific basis for exploitation and utilization of semen plantaginis.
Owner:JILIN UNIV
Biomolecule with therapeutic tumour action and its use
InactiveCN1528776AOvercome toxic side effectsInhibition of telomerase activityOrganic active ingredientsSugar derivativesSide effectUracil nucleoside
The invention is a biomolecule curing tumours, its character: in a special sequence, it is a dichain RNA molecule with 23 basic groups. Its molecular structure contains adenine nucleotide A, guanosine G, cytidine C and uridine U. Its beneficial effects: 1, obvious effect of prohibiting tumour growth and high selectivity, able to overcome poisonous side effect of quinoline drug; 2, good stability; 3, effect amplification; 4, easy to prepare. It can be used to prepare clinical antitumor drug, where the drug form is any one of water solution injection, liposome soliquoid injection and latex.
Owner:TIANJIN SAIER BIOTECH
Synthetic method of 3-acetylpyridine adenine dinucleotide
InactiveCN103601780ALow priceEasy to buySugar derivativesSugar derivatives preparationChemical synthesisMorpholine
The invention discloses a chemical synthesis method of 3-acetylpyridine adenine dinucleotide, comprising the following steps: (1) D-ribose is used as a starting material to prepare monophosphate-3-acetylpyridine-alpha-D-nucleoside; (2) adenosine is used as a main raw material to prepare morpholine adenosine monophosphate; and (3) a docking reaction between morpholine adenosine monophosphate and monophosphate-3-acetylpyridine-alpha-D-nucleoside is carried out to prepare 3-acetylpyridine adenine dinucleotide. According to the chemical synthesis method, a commercial industrial product D-ribose is used as the starting raw material; any enzyme is not used during the preparation process of an intermediate; price of raw materials is low and the raw materials are easy to purchase; and production cost of the product is reduced greatly. In addition, by the chemical synthesis method, yield of the product is high, and total yield of the reaction is 4.2% and is remarkably higher than product yield of a present biosynthesis method.
Owner:BEIJING LEADMAN BIOCHEM
Special medium for rapid propagation of arundo donax and cultivation method of arundo donax
ActiveCN110122334AImprove reproductive efficiencyClimate change adaptationAfforestationMicrobiologyChloride sodium
The invention discloses a special medium for rapid propagation of arundo donax. The medium comprises the following components: an MS basic medium, 6-benzylaminoadenine (6-BA) of 1-8 mg / L, indolebutyric acid (IBA) of 0.2-1.5mg / L, sodium chloride of 50-300mmol / L, and adenine nucleoside triphosphate (ATP) of 0.05-0.5mg / L. According to the special medium, based on a traditional MS basic medium and cytokinin, sodium chloride and ATP are added, and especially that ATP is added in batches, so that the reproductive efficiency of arundo donax can be significantly improved.
Owner:YUNCHENG UNIVERISTY
Adenine single-base editing product without PAM limitation, method and application
PendingCN114560946AWork around the limitation of missing a suitable PAMHydrolasesPeptide/protein ingredientsDiseaseBeta thalassemia
The invention discloses a PAM limitation-free adenine single base editing product, a PAM limitation-free adenine single base editing method and an application of the PAM limitation-free adenine single base editing product. The product comprises a fusion protein, the fusion protein comprises the following two parts: adenine nucleoside deaminase and endonuclease, the amino acid sequence of the adenine nucleoside deaminase comprises a sequence as shown in SEQ ID No.1, and the amino acid sequence of the endonuclease comprises a sequence as shown in SEQ ID No.2. The product can realize the introduction of Agt without PAM limitation into the genes of cells, especially hematopoietic stem / progenitor cells; g, replacement editing is carried out, the edited autologous hematopoietic stem cells of the patient are transfused back, and the purpose of thoroughly curing diseases in a long-acting mode can be achieved. The product is applied to repair of beta-thalassemia (thalassemia) related mutation IVS2-654 Cgt; the beta-globin gene editing efficiency is high, the expression level of the beta-globin gene can be remarkably and effectively improved, and the beta-globin gene editing method has the application potential in clinical treatment of beta-anemia.
Owner:EAST CHINA NORMAL UNIV +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com