Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
38 results about "Carbocyclic nucleoside" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
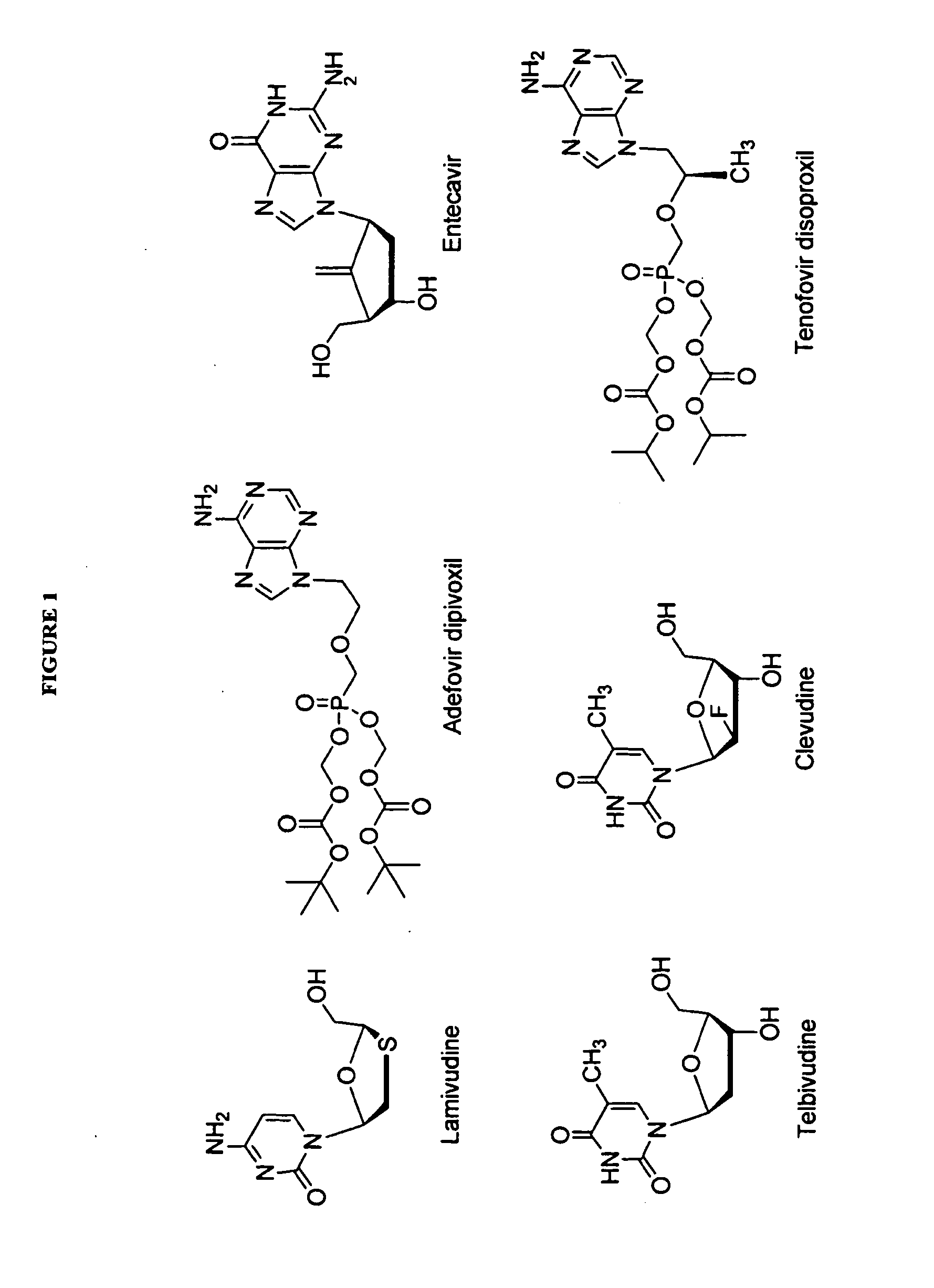
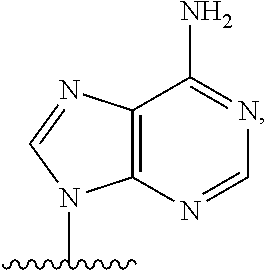
Carbocyclic nucleosides (also referred to as carbanucleosides) are nucleoside analogues in which a methylene group has replaced the oxygen atom of the furanose ring. These analogues have the nucleobase attached at a simple alkyl carbon rather than being part of a hemiaminal ether linkage. As a result, they have increased chemical stability. They also have increased metabolic stability because they are unaffected by phosphorylases and hydrolases that cleave the glycosidic bond between the nucleobase and furanose ring of nucleosides. They retain many of the biological properties of the original nucleosides with respect to recognition by various enzymes and receptors.
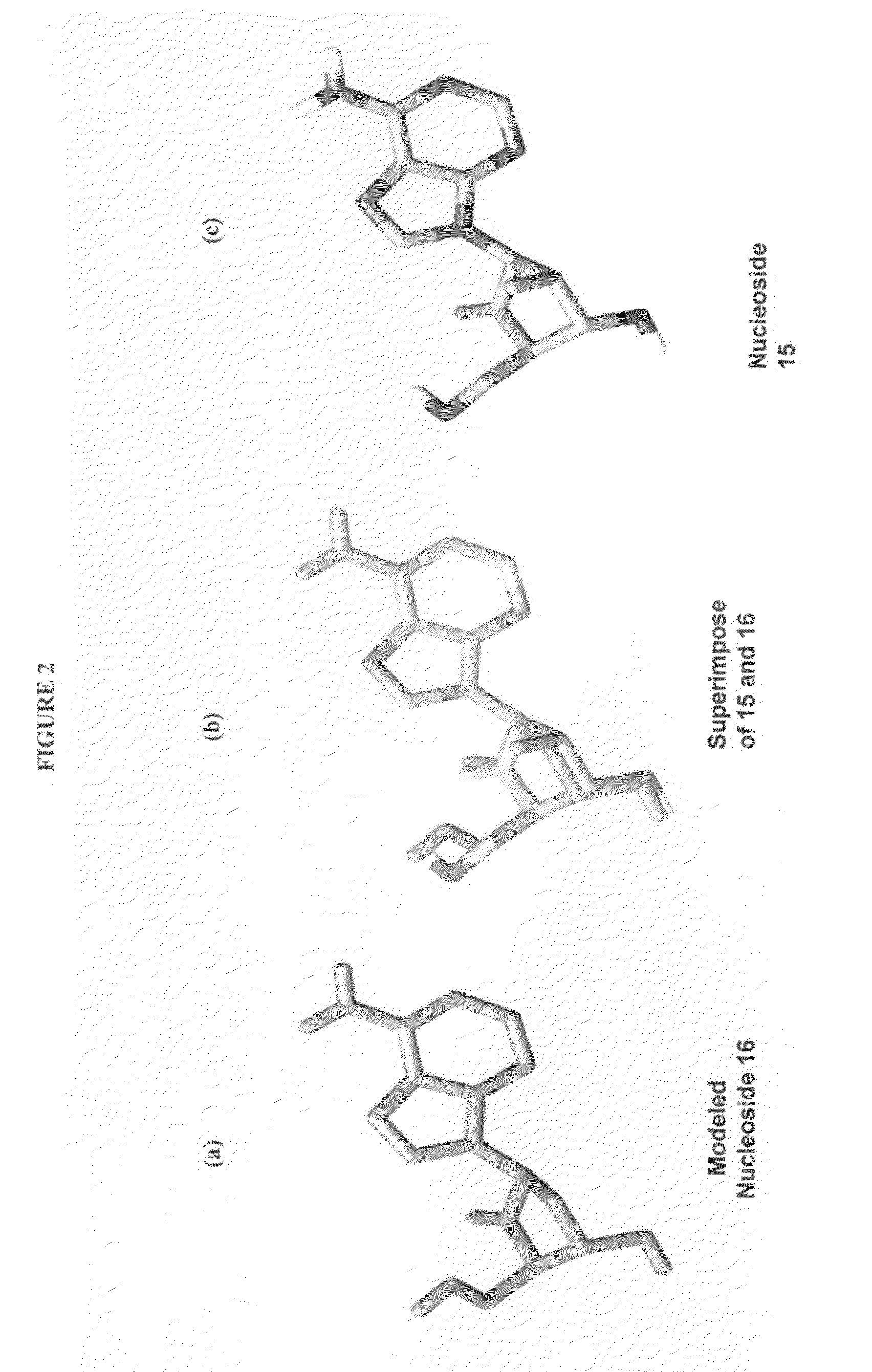
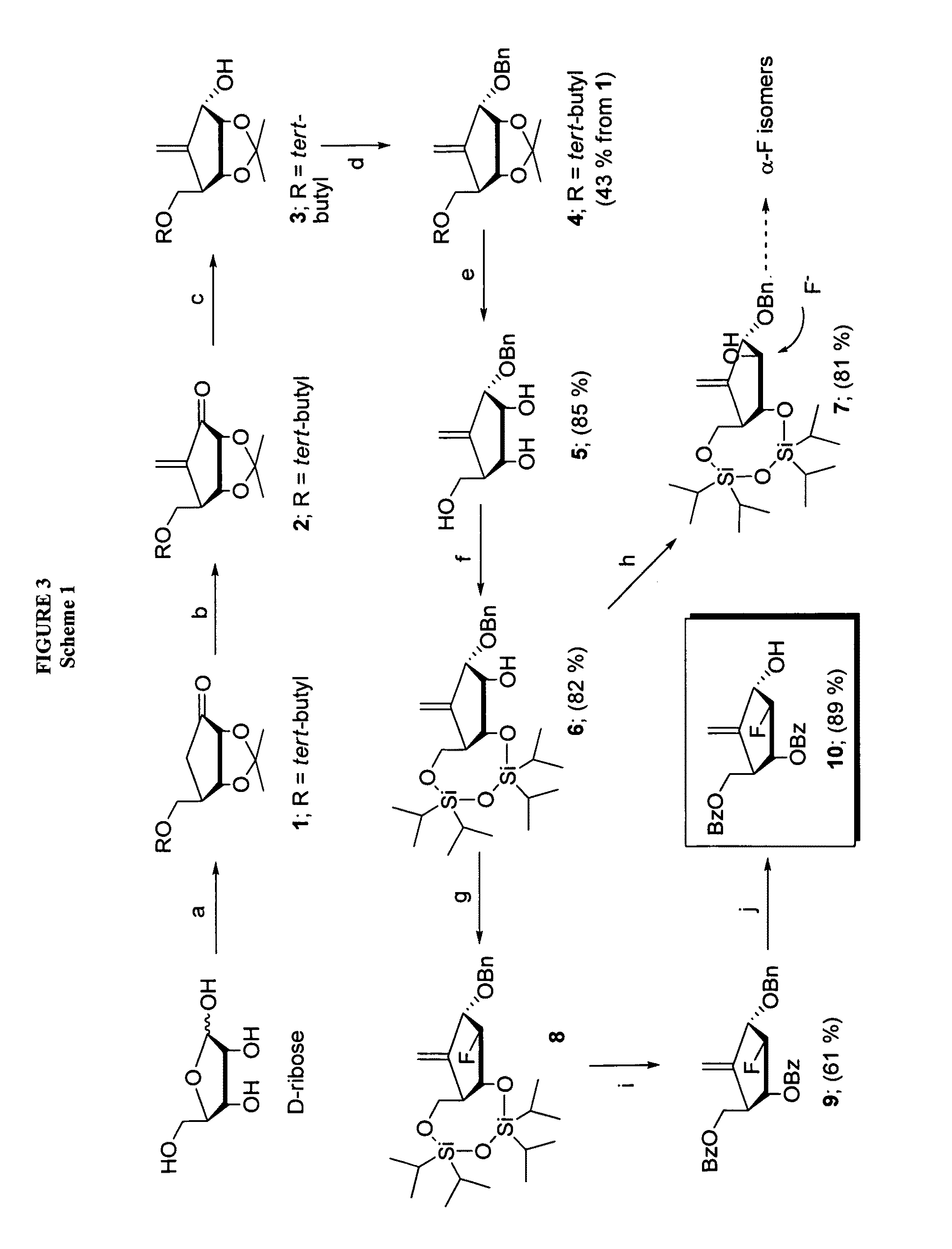
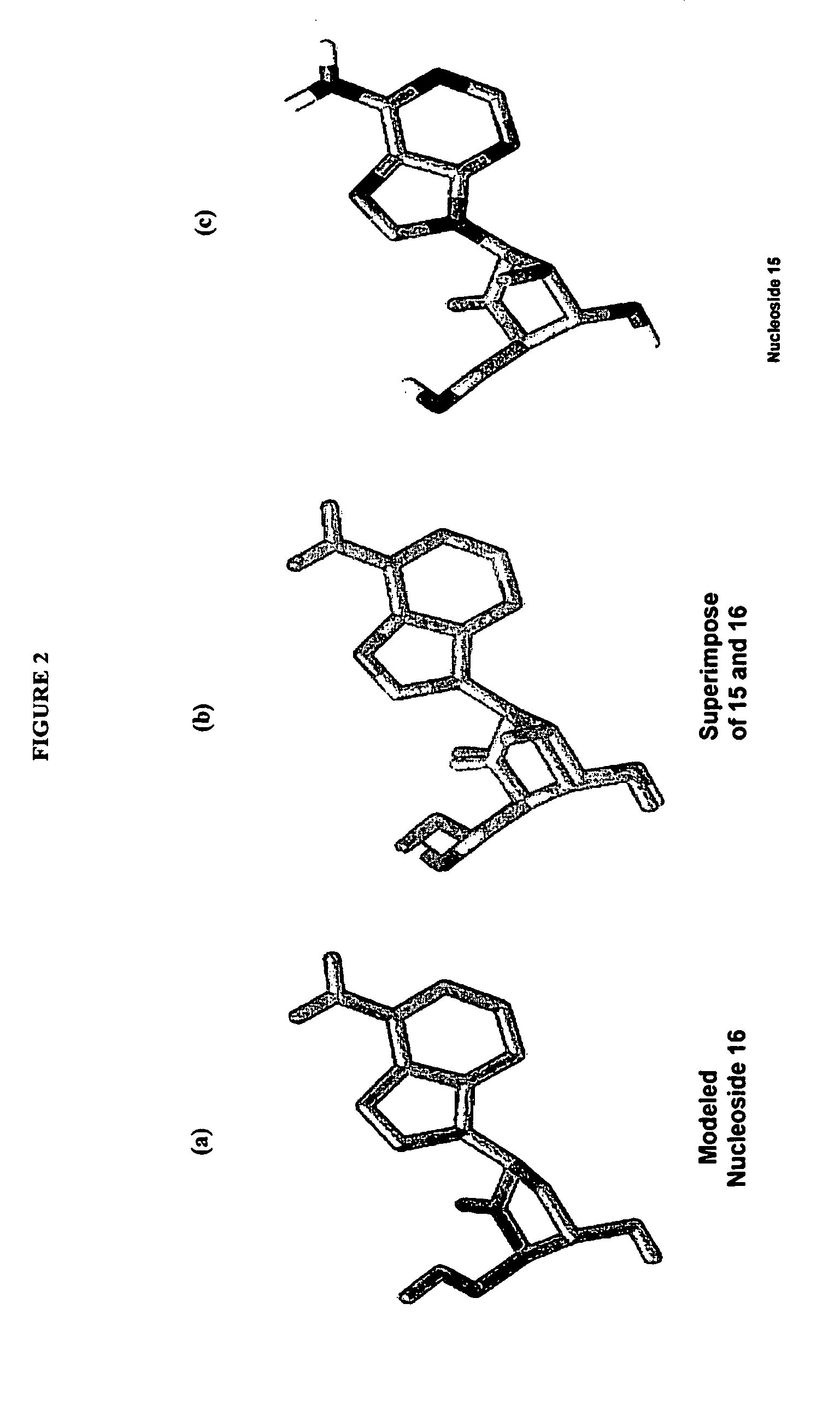
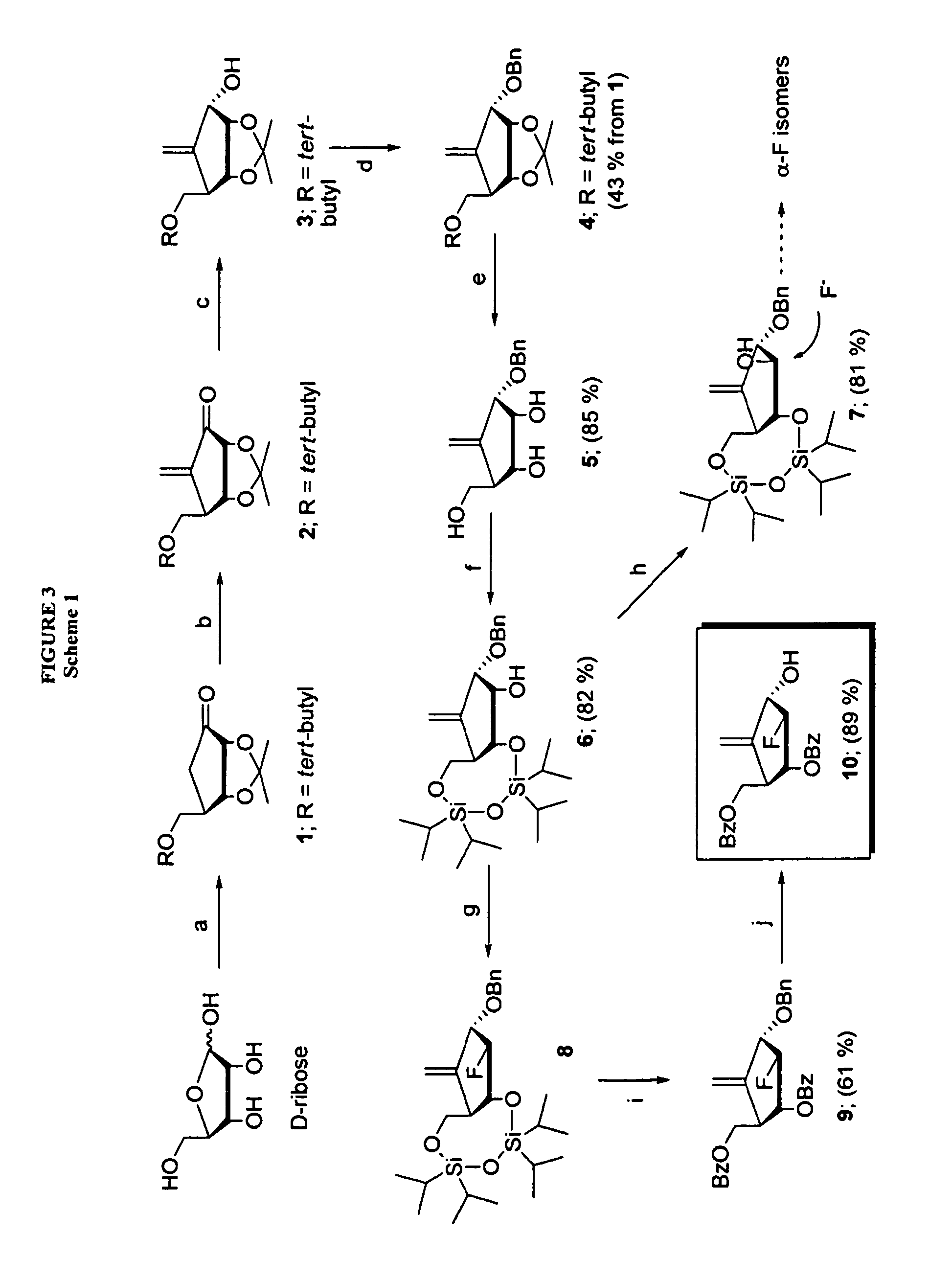
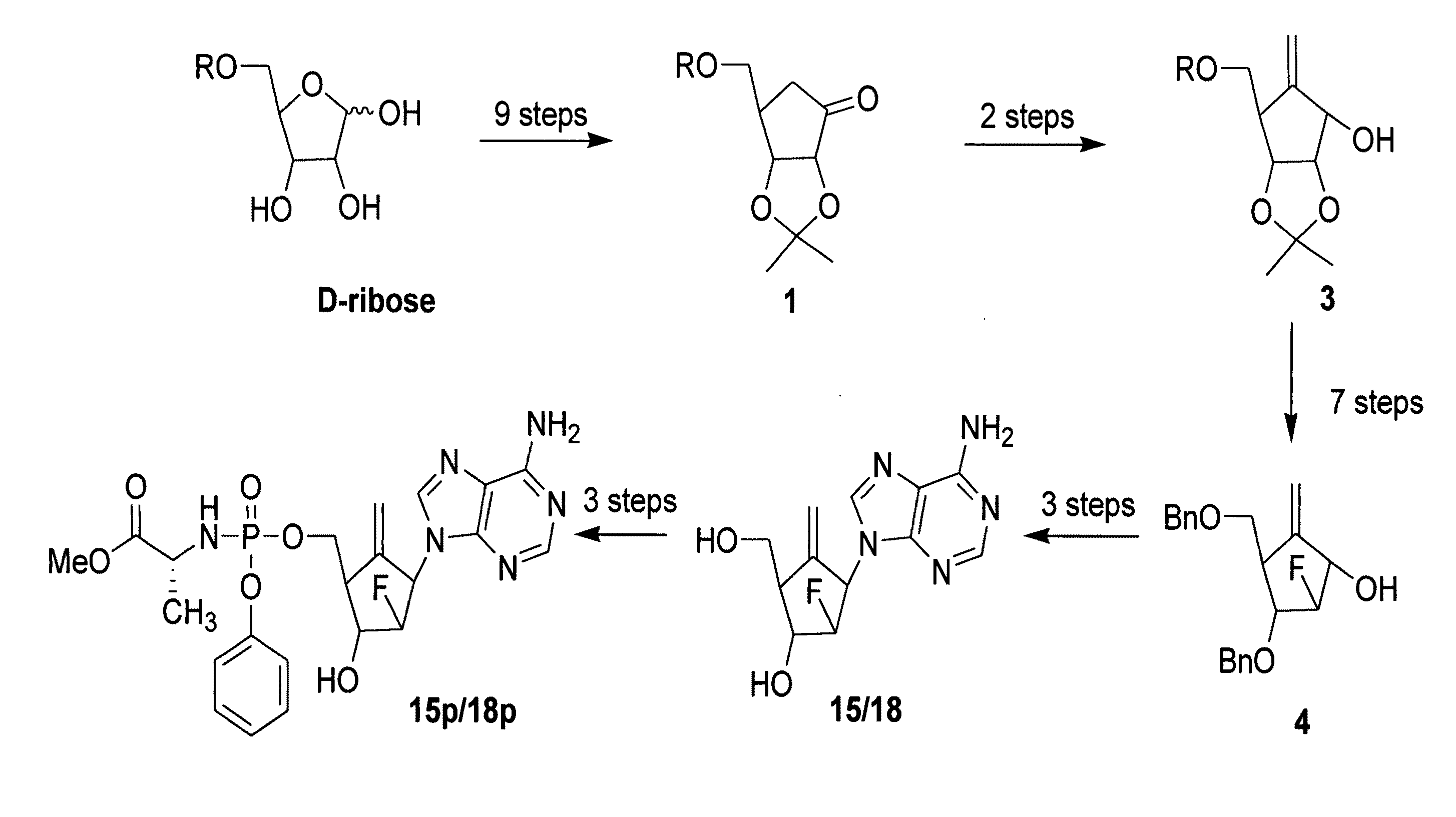
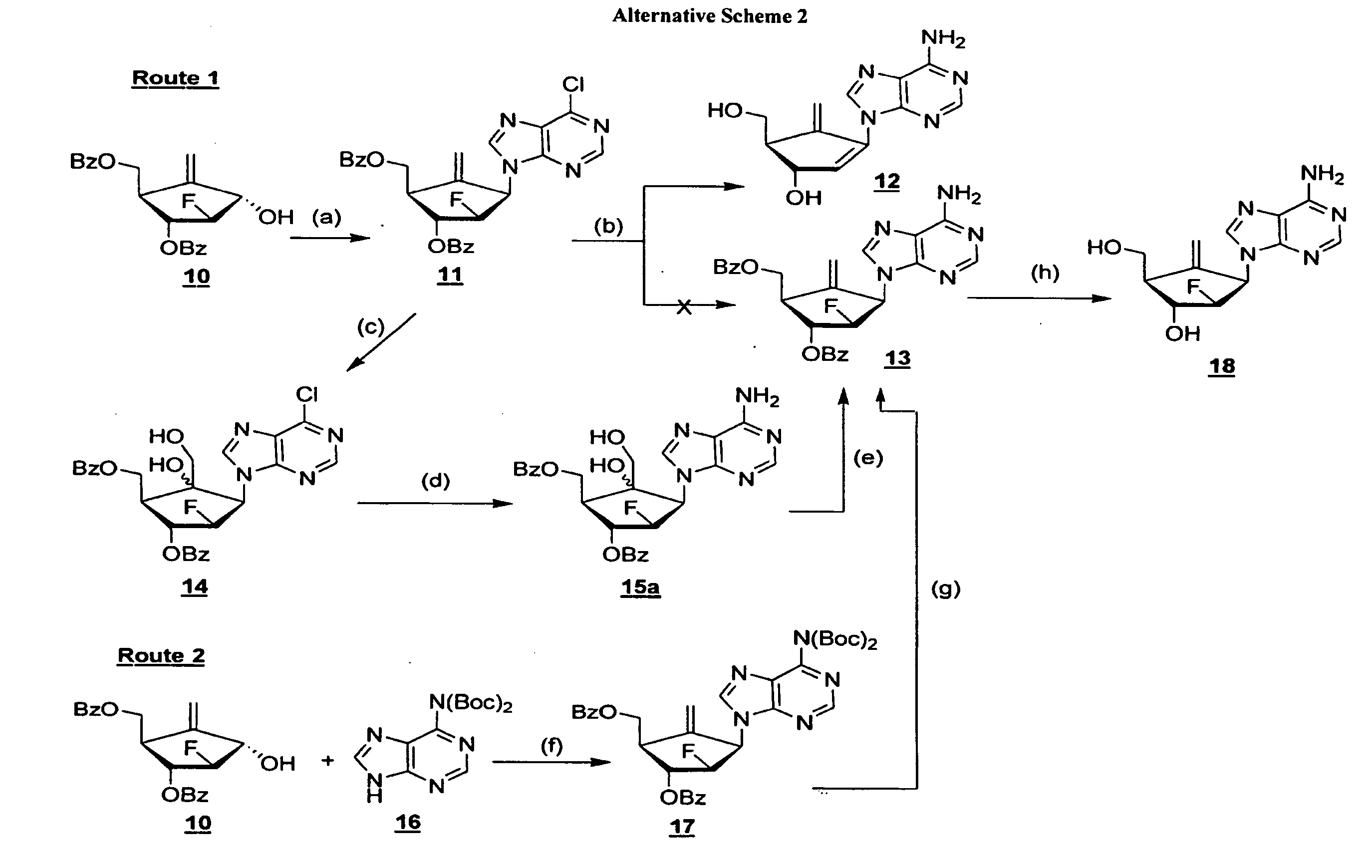
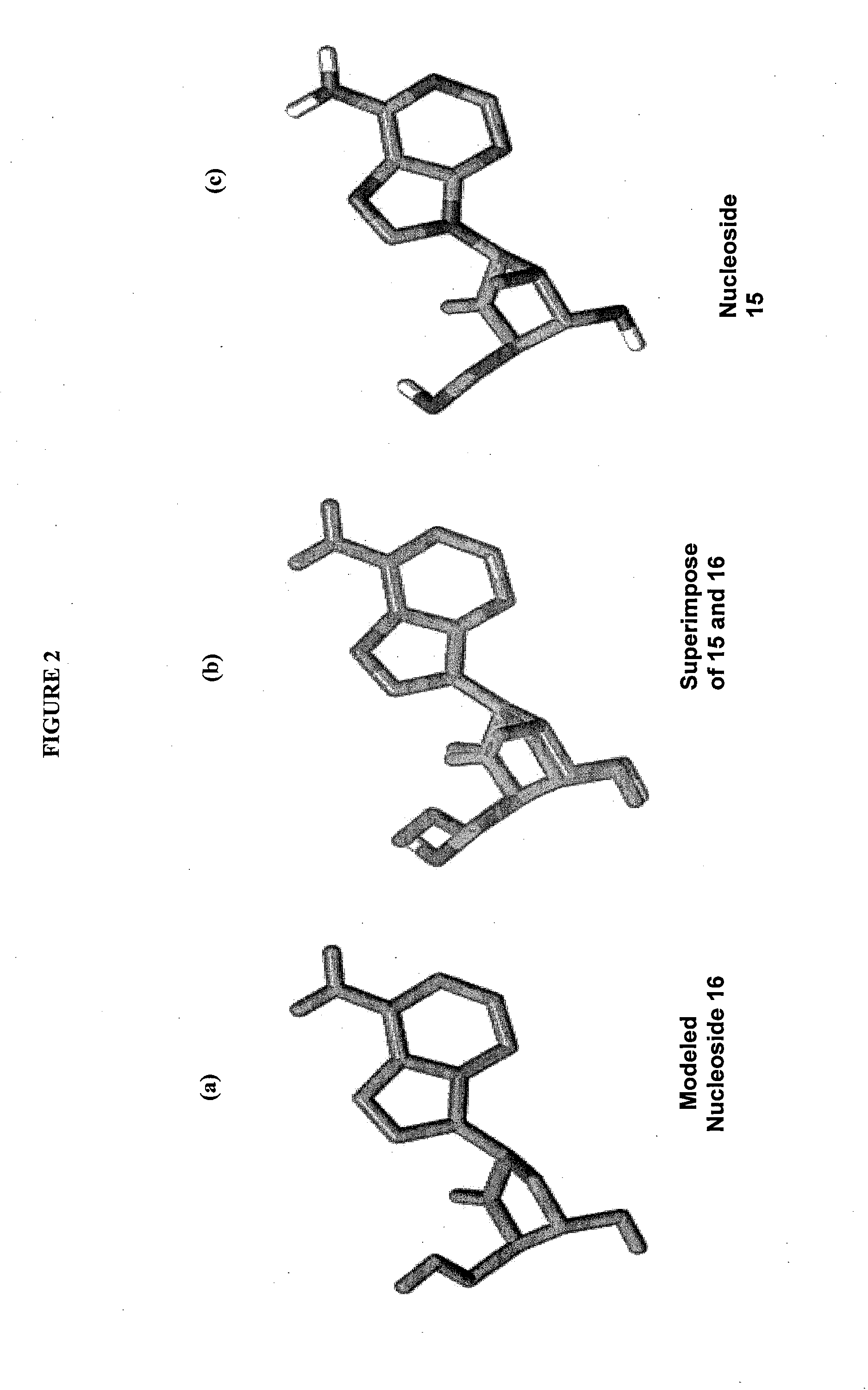
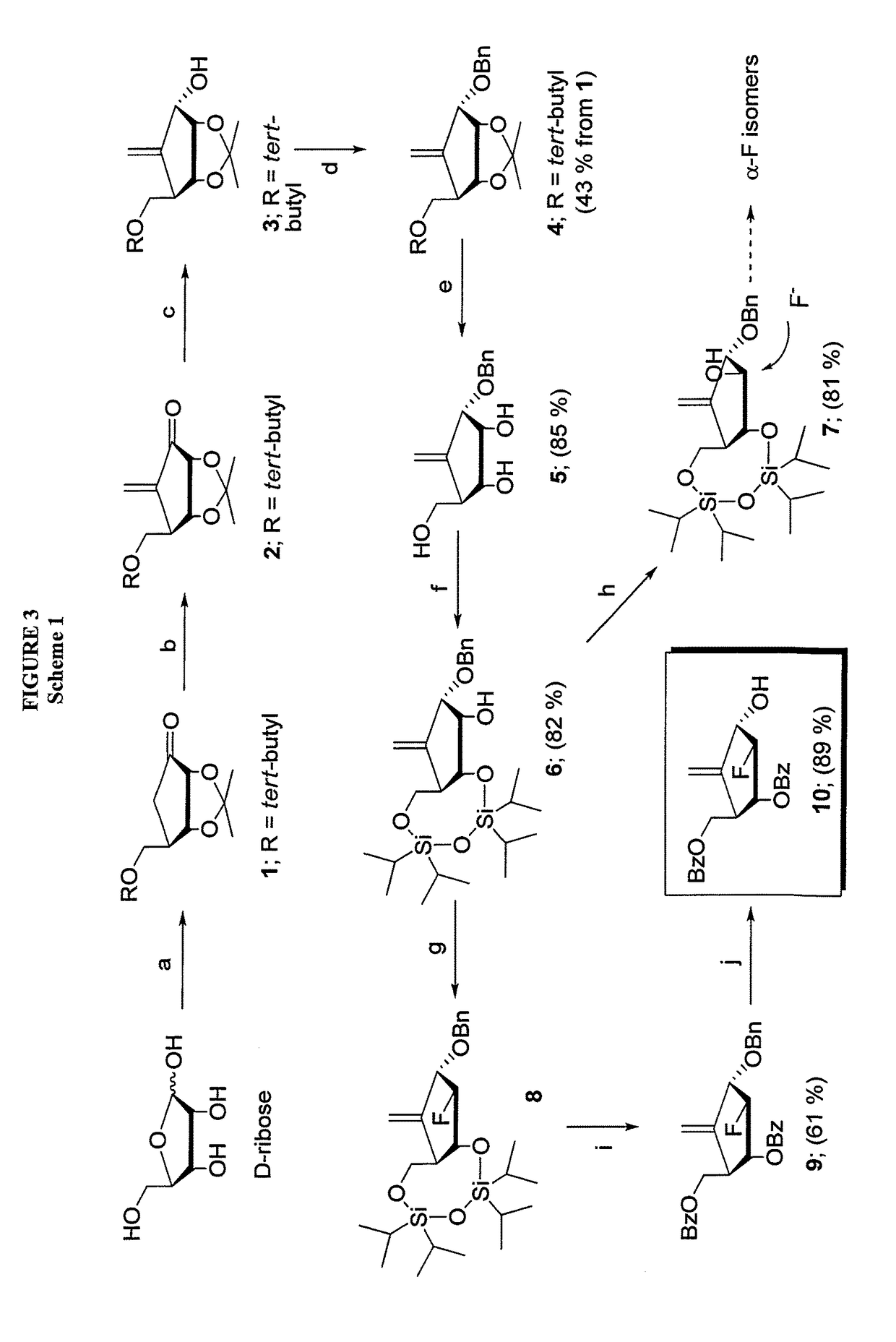
2′-fluoro-6′-methylene carbocyclic nucleosides and methods of treating viral infections
The present invention relates to 2′-Fluoro-6′-methylene carbocyclic nucleosides, pharmaceutical compositions containing these nucleosides and their use in the treatment or prophylaxis of a number of viral infections and secondary disease states and conditions thereof, especially including Hepatitis B virus (HBV) and secondary disease states and conditions thereof (cirrhosis and liver cancer), Heptatitis C virus (HCV), Herpes Simplex virus I and II (HSV-1 and HSV-2), cytomegalovirus (CMV), Varicella-Zoster Virus (VZV) and Epstein Barr virus (EBV) and secondary cancers which occur thereof (lymphoma, nasopharyngeal cancer, including drug resistant (especially including lamivudine and / or adefovir resistant) and other mutant forms of these viruses.
Owner:UNIV OF GEORGIA RES FOUND INC
2′-fluoro-6′methylene carbocyclic nucleosides and methods of treating viral infections
ActiveUS8946244B2Reduce the possibilityBiocideSugar derivativesHerpes zoster virusNasopharyngeal cancer
The present invention relates to 2′-Fluoro-6′-methylene carbocyclic nucleosides, pharmaceutical compositions containing these nucleosides and their use in the treatment or prophylaxis of a number of viral infections and secondary disease states and conditions thereof, especially including Hepatitis B virus (HBV) and secondary disease states and conditions thereof (cirrhosis and liver cancer), Hepatitis C virus (HCV), Herpes Simplex virus I and II (HSV-1 and HSV-2), cytomegalovirus (CMV), Varicella-Zoster Virus (VZV) and Epstein Barr virus (EBV) and secondary cancers which occur thereof (lymphoma, nasopharyngeal cancer, including drug resistant (especially including lamivudine and / or adefovir resistant) and other mutant forms of these viruses.
Owner:UNIV OF GEORGIA RES FOUND INC
2'-Fluoro-6'-Methylene Carbocyclic Nucleosides and Methods of Treating Viral Infections
The present invention relates to 2′-Fluoro-6′-methylene carbocyclic nucleosides, pharmaceutical compositions containing these nucleosides and their use in the treatment or prophylaxis of a number of viral infections and secondary disease states and conditions thereof, especially including Hepatitis B virus (HBV) and secondary disease states and conditions thereof (cirrhosis and liver cancer), Heptatitis C virus (HCV), Herpes Simplex virus I and II (HSV-1 and HSV-2), cytomegalovirus (CMV), Varicella-Zoster Virus (VZV) and Epstein Barr virus (EBV) and secondary cancers which occur thereof (lymphoma, nasopharyngeal cancer, including drug resistant (especially including lamivudine and / or adefovir resistant) and other mutant forms of these viruses.
Owner:UNIV OF GEORGIA RES FOUND INC
2'-fluoro-6'-methylene carbocyclic nucleosides and methods of treating viral infections
ActiveUS20130005677A1Reduce infectious virus titerReduce cell viabilityBiocideSugar derivativesHerpes simplex diseaseCirrhosis
The present invention relates to 2′-Fluoro-6′-methylene carbocyclic nucleosides, pharmaceutical compositions containing these nucleosides and their use in the treatment or prophylaxis of a number of viral infections and secondary disease states and conditions thereof, especially including Hepatitis B virus (HBV) and secondary disease states and conditions thereof (cirrhosis and liver cancer), Heptatitis C virus (HCV), Herpes Simplex virus I and II (HSV-1 and HSV-2), cytomegalovirus (CMV), Varicella-Zoster Virus (VZV) and Epstein Barr virus (EBV) and secondary cancers which occur thereof (lymphoma, nasopharyngeal cancer, including drug resistant (especially including lamivudine and / or adefovir resistant) and other mutant forms of these viruses.
Owner:UNIV OF GEORGIA RES FOUND INC
D-amino-acid ester-containing nucleoside amino phosphonate derivative and medical purpose thereof
ActiveCN106188192AImprove biological activityGood metabolic stabilityOrganic active ingredientsSugar derivativesFuranPhosphate
The invention relates to a novel nucleoside phosphate / phosphonate prodrug containing non-naturalD-amino acid ester, a preparation method and a medical purpose thereof. The novel nucleoside phosphate / phosphonate prodrug containing a substituted benzyl group is a compound shown in a formula (I) or a formula (II) or its isomer or medicinal salt, which can be used as the prodrug of various nucleoside analogues such as acyclic nucleoside, carbocyclic nucleoside, and furan ring nucleoside, biological activity of the nucleoside compound can be enhanced, so that the derivative can be used for treating virus infection and cancer.
Owner:SHENZHEN VYBIO PHARM TECH CO LTD
Method for synthesis of chiral five-membered carbocyclic purine nucleoside by asymmetric [3+2] cyclization reaction
ActiveCN107698590AHigh stereoselectivitySimple structureAsymmetric synthesesPurineEnantio selectivity
Belonging to the field of asymmetric synthesis in organic chemistry, the invention discloses a method for synthesis of chiral five-membered carbocyclic purine nucleoside by asymmetric [3+2] cyclization reaction. The method includes: taking alpha-purine substituted acrylic ester and MBH carbonic ester as the raw materials, adopting chiral SITCP as the catalyst, and carrying out reaction to obtain achiral five-membered carbocyclic nucleoside compound. The reaction has good diastereoselectivity and enantioselectivity, and the yield is up to 93%.
Owner:HENAN NORMAL UNIV
Preparation and medicine purpose of nucleoside alkoxide benzyl phosphoramidic acid/phosphonate derivative
The invention relates to a preparation method and a purpose of a novel nucleoside phosphoramidic acid / phosphonate prodrug simultaneously formed by alkoxide benzyl alcohol and D or L-amino-acid ester. The novel nucleoside phosphoramidic acid / phosphonate prodrug containing alkoxide benzyl is a compound shown by the formula (I) or an isomer or a medicinal salt of the compound. The compound can be used as prodrug of various nucleoside analogs such as acyclic nucleoside, carbocyclic nucleoside and furan ring nucleoside; the bioactivity of the nucleoside compounds are enhanced, so that the application of the compound in the fields of virus infection and cancer treatment is optimized. The formula is shown in the description.
Owner:刘沛
Method for synthesizing chiral pentabasic carbocyclic nucleoside analog by asymmetric [3+2] cycloaddition
The invention discloses a method for synthesizing a chiral pentabasic carbocyclic nucleoside analog by asymmetric [3+2] cycloaddition. The reaction equation is as shown in the specification. According to the method for synthesizing a chiral pentabasic carbocyclic nucleoside analog by asymmetric [3+2] cycloaddition, provided by the invention, a specific chiral catalyst is used, so that the chiral product can be obtained at a high yield and a high enantiomer excess value; besides, the reaction has the advantages that the operation is simple, the reaction condition is mild and the catalyst is inexpensive and easily obtainable, and a simple and practical synthetic method is provided for synthesis of chiral pentabasic carbocyclic nucleoside analogues.
Owner:HENAN NORMAL UNIV
2'-fluoro-6'-methylene carbocyclic nucleosides and methods of treating viral infections
The present invention relates to 2′-Fluoro-6′-methylene carbocyclic nucleosides, pharmaceutical compositions containing these nucleosides and their use in the treatment or prophylaxis of a number of viral infections and secondary disease states and conditions thereof, especially including Hepatitis B virus (HBV) and secondary disease states and conditions thereof (cirrhosis and liver cancer), Heptatitis C virus (HCV), Herpes Simplex virus I and II (HSV-1 and HSV-2), cytomegalovirus (CMV), Varicella-Zoster Virus (VZV) and Epstein Barr virus (EBV) and secondary cancers which occur thereof (lymphoma, nasopharyngeal cancer, including drug resistant (especially including lamivudine and / or adefovir resistant) and other mutant forms of these viruses, especially HBV.
Owner:UNIV OF GEORGIA RES FOUND INC
Method for synthesizing chiral ternary carbocyclic nucleoside through asymmetric cyclopropanation triggered by Michael addition
The invention discloses a method for synthesizing chiral ternary carbocyclic nucleoside through asymmetric cyclopropanation triggered by Michael addition and belongs to the field of asymmetric synthesis in organic chemistry. Chiral cyclopropane carbocycle purine nucleoside is prepared from alpha-purine substituted acrylate and bromo-tert-butyl acetate as raw materials after a chiral amine catalysis reaction derived by quinine, the reaction enantioselectivity is good, and the yield is medium to excellent.
Owner:HENAN NORMAL UNIV
2′-fluoro-6′-methylene carbocyclic nucleosides and methods of treating viral infections
The present invention relates to 2′-Fluoro-6′-methylene carbocyclic nucleosides, pharmaceutical compositions containing these nucleosides and their use in the treatment or prophylaxis of a number of viral infections and secondary disease states and conditions thereof, especially including Hepatitis B virus (HBV) and secondary disease states and conditions thereof (cirrhosis and liver cancer), Hepatitis C virus (HCV), Herpes Simplex virus I and II (HSV-1 and HSV-2), cytomegalovirus (CMV), Varicella-Zoster Virus (VZV) and Epstein Barr virus (EBV) and secondary cancers which occur thereof (lymphoma, nasopharyngeal cancer, including drug resistant (especially including lamivudine and / or adefovir resistant) and other mutant forms of these viruses, especially HBV.
Owner:UNIV OF GEORGIA RES FOUND INC
2'3'-cyclic dinucleotides comprising carbocyclic nucleotide
InactiveUS20190359645A1Organic active ingredientsSugar derivativesPyrimidine NucleotidesBiochemistry
The present disclosure relates to 2′3′-cyclic dinucleotides comprising a carbocyclic nucleotide and derivatives thereof, that can modulate the activity of the STING adaptor protein.
Owner:INST OF ORGANIC CHEM & BIOCHEM V V I
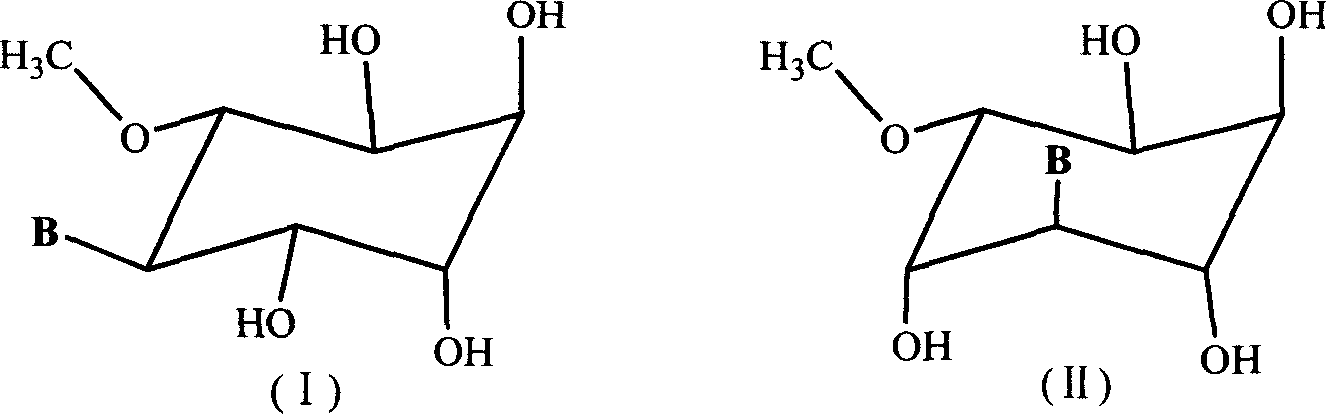
A group of six-carbocycle nucleoside analogue, its synthesis method and antiviral application
InactiveCN1803819ASaccharide with heterocyclic radicalsOrganic active ingredientsSodium bicarbonate5-fluorocytidine
The disclosed six-membered carbocyclic nucleoside analogues comprise: adenosine analogue, guanosine analogue, carnine analogue, mercaptopurine riboside analogue, cytidine analogue, 5-fluorocytidine analogue, uridine analogue, 5-fluorouridine analogue, and thymidine analogue as well as their acceptable salts formed by equimolar acid in pharmacy. Wherein, the opposite five-step synthesis method using the pinitol, acetone, methane sulfonyl chloride, p-toluenesulfonyl chloride, benzene sulfochloride, and nucleoside base as materials; the pyridine, water, glacial acetic acid, absolute methanol, DMSO, N, N-DMF as the solvent; the p-toluenesulfonic acid, 2, 2-dimethoxylpropane, anhydrous NaSO4, NaHCO3, triethylamine, and anhydrous K2CO3 as the catalyst. This invention restrains specially the replication of HIV and herpesvirus.
Owner:SHANDONG UNIV
(-)-[gamma]-lactamase, gene, mutant, vector as well as preparation method and application of (-)-[gamma]-lactamase
ActiveCN105950595AHigh optical activityMild reaction conditionsHydrolasesFermentationMutantSubstrate concentration
The invention relates to (-)-[gamma]-lactamase, a gene and a mutant of the (-)-[gamma]-lactamase, a recombinant expression plasmid and a recombinant expression transformant containing the gene and the mutant, a preparation method of the (-)-[gamma]-lactamase and an application of the (-)-[gamma]-lactamase in preparing (+)-[gamma]-lactam. Compared with the prior art, the (-)-[gamma]-lactamase disclosed by the invention has the characteristics of being high in enzymatic activity and good in substrate concentration tolerance; the (+)-[gamma]-lactam, which is prepared through enzymatic catalysis, has the advantages of being mild in reaction condition, high in substrate concentration, low in catalyst dosage and the like; therefore, the (-)-[gamma]-lactamase has a good application prospect in industrial production of the (-)-[gamma]-lactamase which is an intermediate product of carbocyclic nucleoside drugs.
Owner:EAST CHINA UNIV OF SCI & TECH
Method for synthesizing chiral non-cyclic and carbocyclic nucleoside analogues
ActiveCN107501266ARaw materials are easy to getEasy to operateOrganic chemistryThermal insulationOrganic synthesis
The invention discloses a method for synthesizing chiral non-cyclic and carbocyclic nucleoside analogues through asymmetric allyl ammoniation and belongs to the technical field of organic synthesis. The method comprises the steps as follows: a reaction solvent, a purine compound 1, an additive, an SKP diphosphine ligand and metal palladium are mixed, an MBH adduct 2 is added, a mixture is subjected to a thermal insulation reaction, an N-allyl olefination product 3 is obtained, and non-cyclic nucleoside 7 is obtained through DIBAL-H reduction or carbocyclic nucleoside 9 is obtained through Grubb olefin metathesis and DIBAL-H reduction in sequence. The method has the advantages of adopting available raw materials and mild reaction conditions and being simple to operate and high in selectivity, and has potential actual application value.
Owner:HENAN NORMAL UNIV
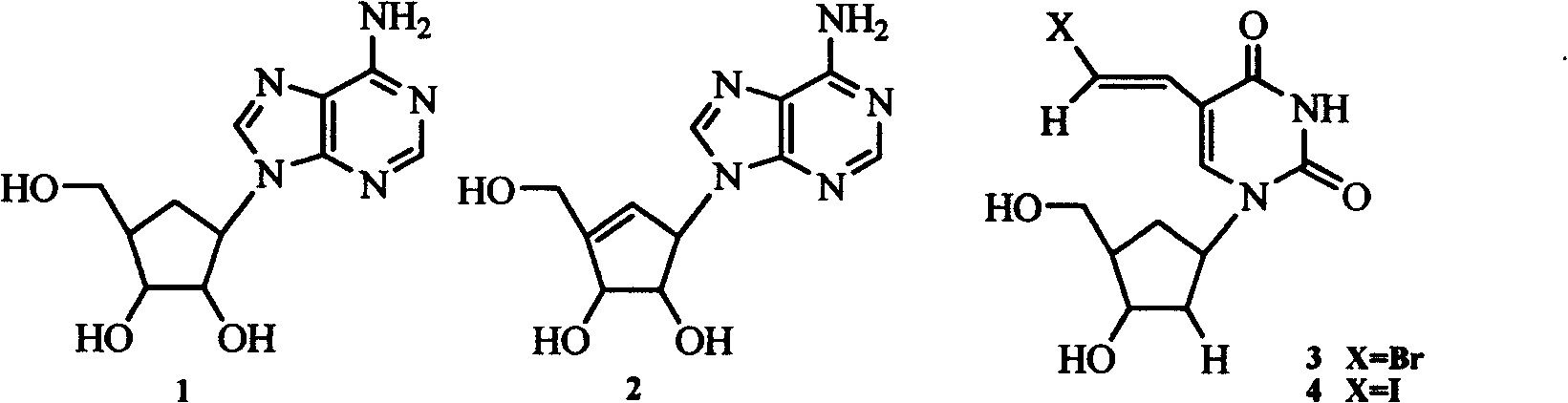
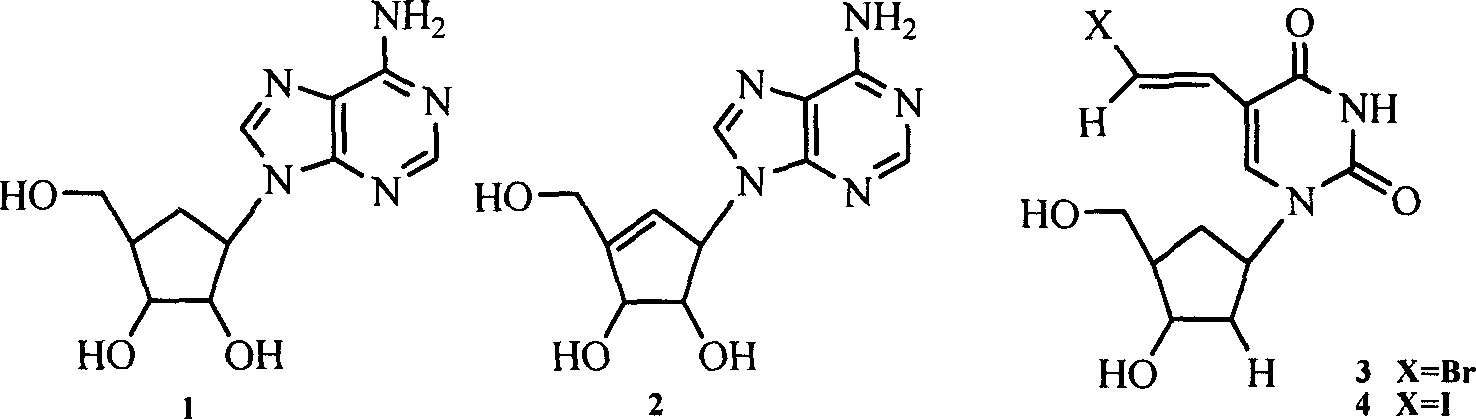
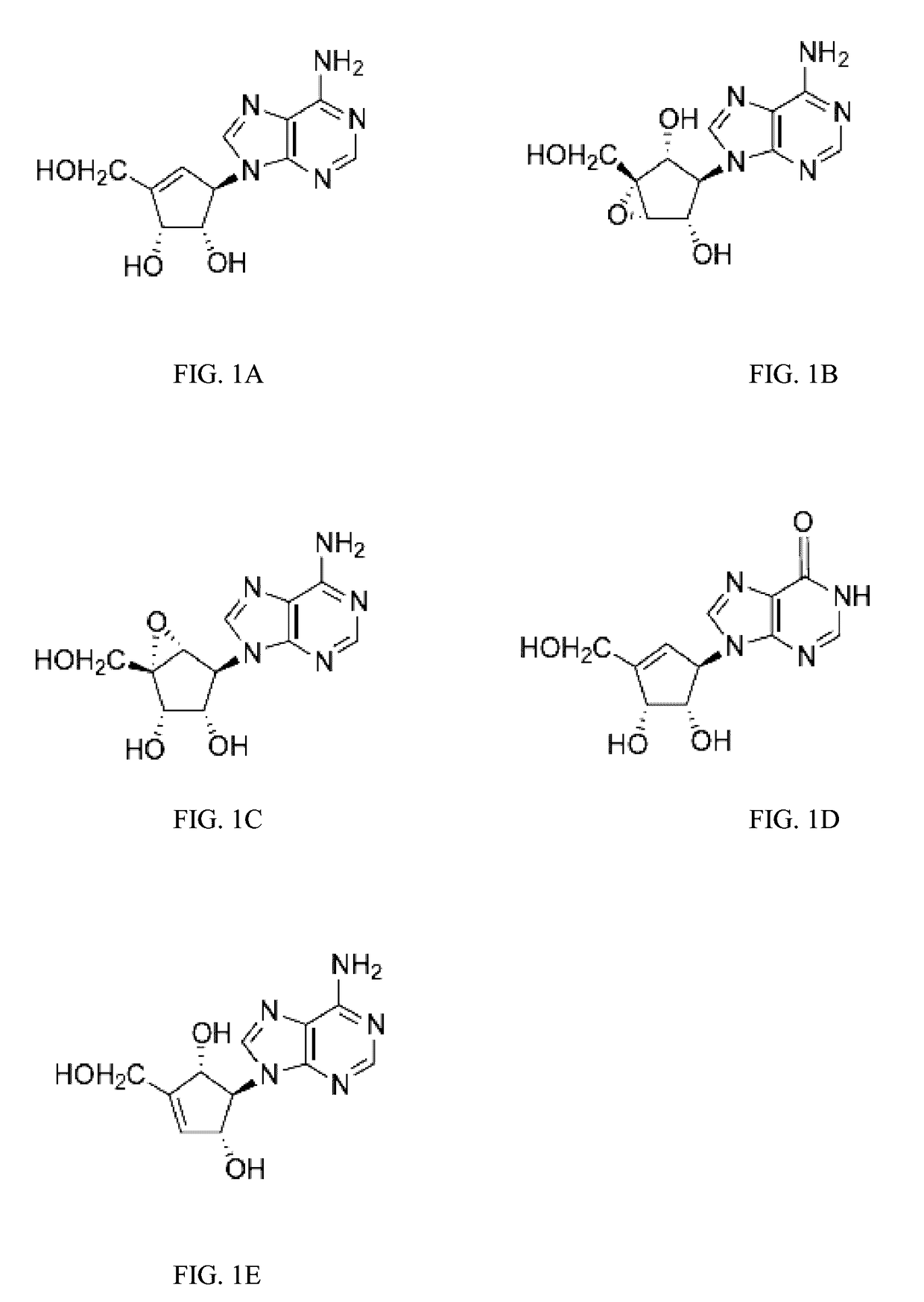
Enantiomers of the 1′,6′-isomer of neplanocin A
Enantiomers of 1′,6′-isoneplanocin, including derivatives of the enantiomers of 1′,6′-isoneplanocin, are disclosed along with novel synthetic methods. In particular, a substituted cyclopentane epoxide is synthesized into the enantiomers of 1′,6′-isoneplanocin. Enantiomers of carbocyclic nucleoside analogs of 3-deazaneplanocin to provide D- and L-like 1′,6′-iso-3-deazaneplanocin are also disclosed. The small molecule chemotherapeutic compounds beneficially provide DNA and RNA antiviral activity, demonstrating activity towards, for example, human cytomegalovirus, measles, Ebola, norovirus, dengue, vaccinia and HBV. Compounds exhibiting reduced S-adenosylhomocysteine hydrolase inhibitory effects are disclosed and provide improved toxicity profiles in comparison to neplanocin. The invention provides improved prophylactic and / or therapeutic antiviral efficacy.
Owner:AUBURN UNIV
Method for synthesizing chiral ternary purine carbocyclic nucleoside analogue through intramolecular asymmetric cycloaddition
InactiveCN105693725ARaw materials are easy to getEasy to operateOrganic chemistryEnantiomerSynthesis methods
The invention discloses a method for synthesizing chiral three-membered purine carbocyclic nucleoside analogues through intramolecular asymmetric cycloaddition. Specifically, the N-9 intramolecular diazonium nucleoside is used as a raw material, through intramolecular asymmetry Cycloaddition Synthesis of Chiral Three-membered Purine Carbocyclic Nucleoside Analogues. In the present invention, the raw materials are easy to obtain, and the operation is simple. By using specific chiral catalysts and reaction conditions, the reaction can obtain chiral products with high yield and high enantiomeric excess value, and the reaction can be achieved in gram-level, still up to High yields and high enantiomeric excess values are maintained. This reaction has several advantages such as novel and easy-to-obtain raw materials, mild reaction conditions, and cheap and easy-to-obtain catalysts. It provides a simple and practical synthetic method for the synthesis of chiral three-membered purine carbocyclic nucleoside analogues.
Owner:HENAN NORMAL UNIV
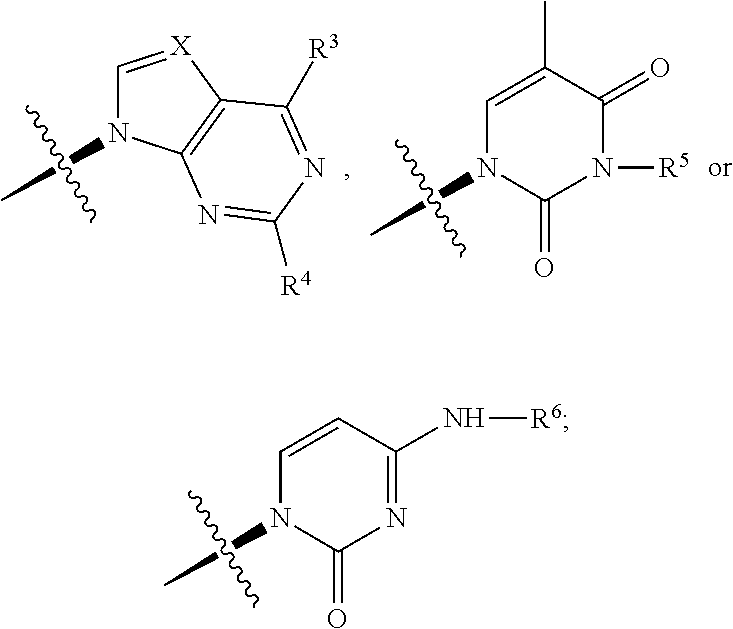
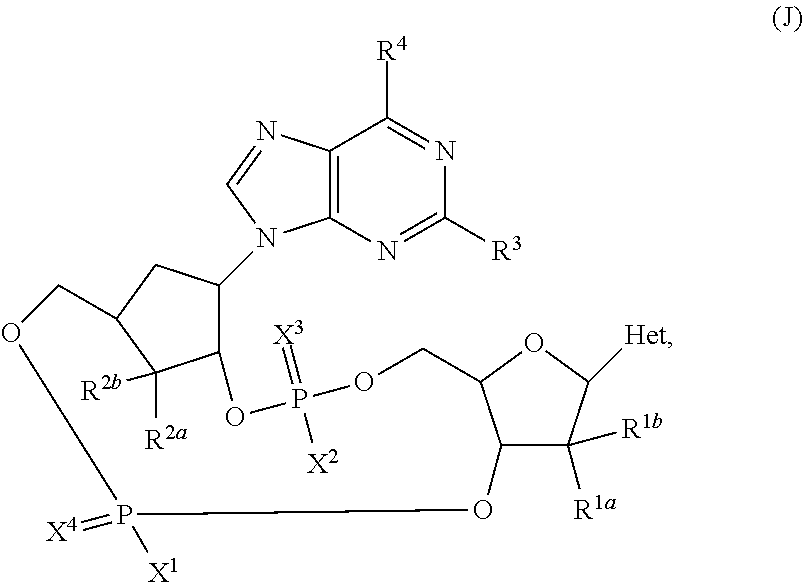
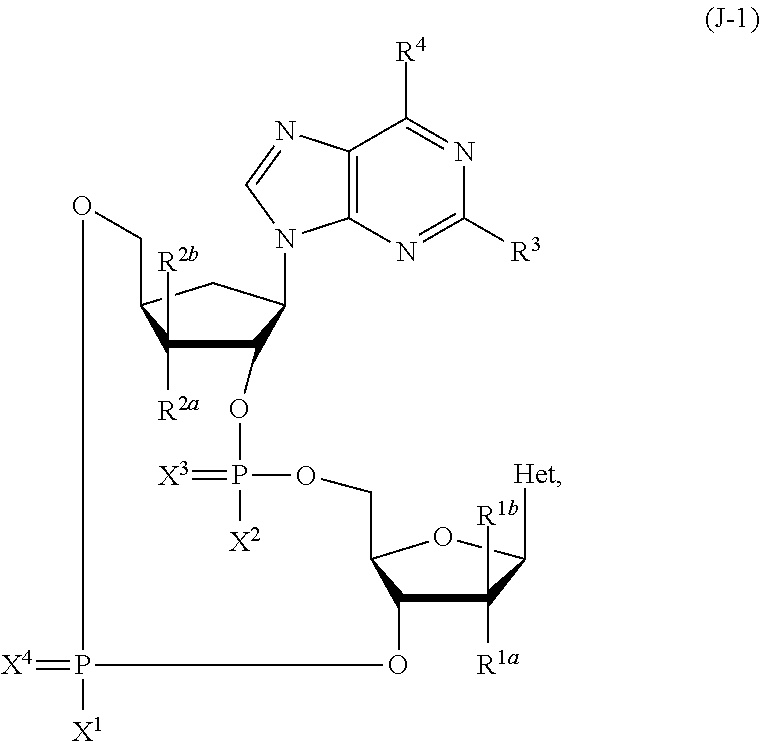
Carbocyclic nucleoside reverse transcriptase inhibitors
ActiveUS20190177326A1Group 5/15 element organic compoundsHeterocyclic compound active ingredientsNucleoside Reverse Transcriptase InhibitorCarbocyclic nucleoside
The present invention is directed carbocyclic nucleoside reverse transcriptase inhibitors compounds of Formula Iand their use in the inhibition of HIV reverse transcriptase, the prophylaxis of infection by HIV, the treatment of infection by HIV, and the prophylaxis, treatment, and delay in the onset or progression of AIDS and / or ARC.
Owner:MERCK SHARP & DOHME LLC +1
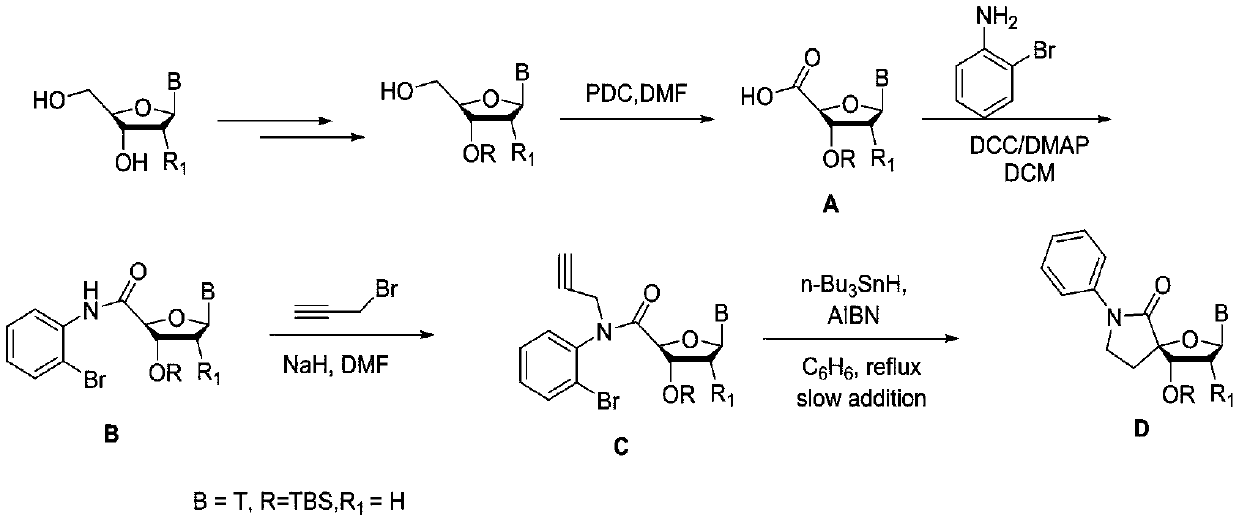
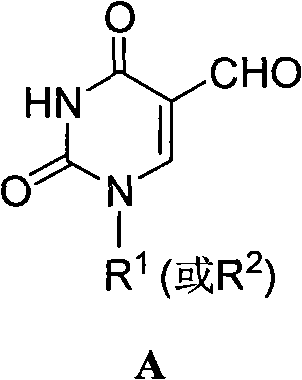
5-formacylpyrimidine carbocyclic nucleoside and preparation method thereof
InactiveCN102603652ARich reactivityThe operation process is simple and convenientOrganic chemistryBulk chemical productionProtecting groupOrganic chemistry
The invention discloses a 5-formacylpyrimidine carbocyclic nucleoside and a preparation method thereof. The compound disclosed by the invention is an important intermediate for synthesizing a novel 5-substituted pyrimidine carbocyclic nucleoside medicine in a more complex structure. A 5-methylpyrimidine carbocyclic nucleoside compound, which is used as a raw material, is subjected to hydroxy protection, methyl oxidation, hydroxy protecting group removal and the like to obtain the 5-formacylpyrimidine carbocyclic nucleoside derivative. The invention has the advantages of simple and safe operational process and mild reaction conditions, and is convenient for industrial production.
Owner:HENAN NORMAL UNIV
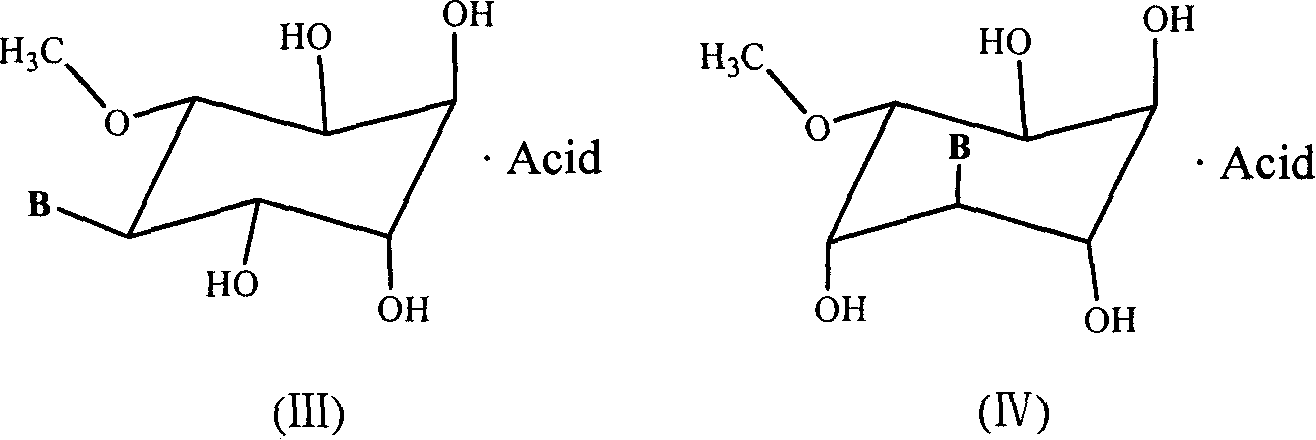
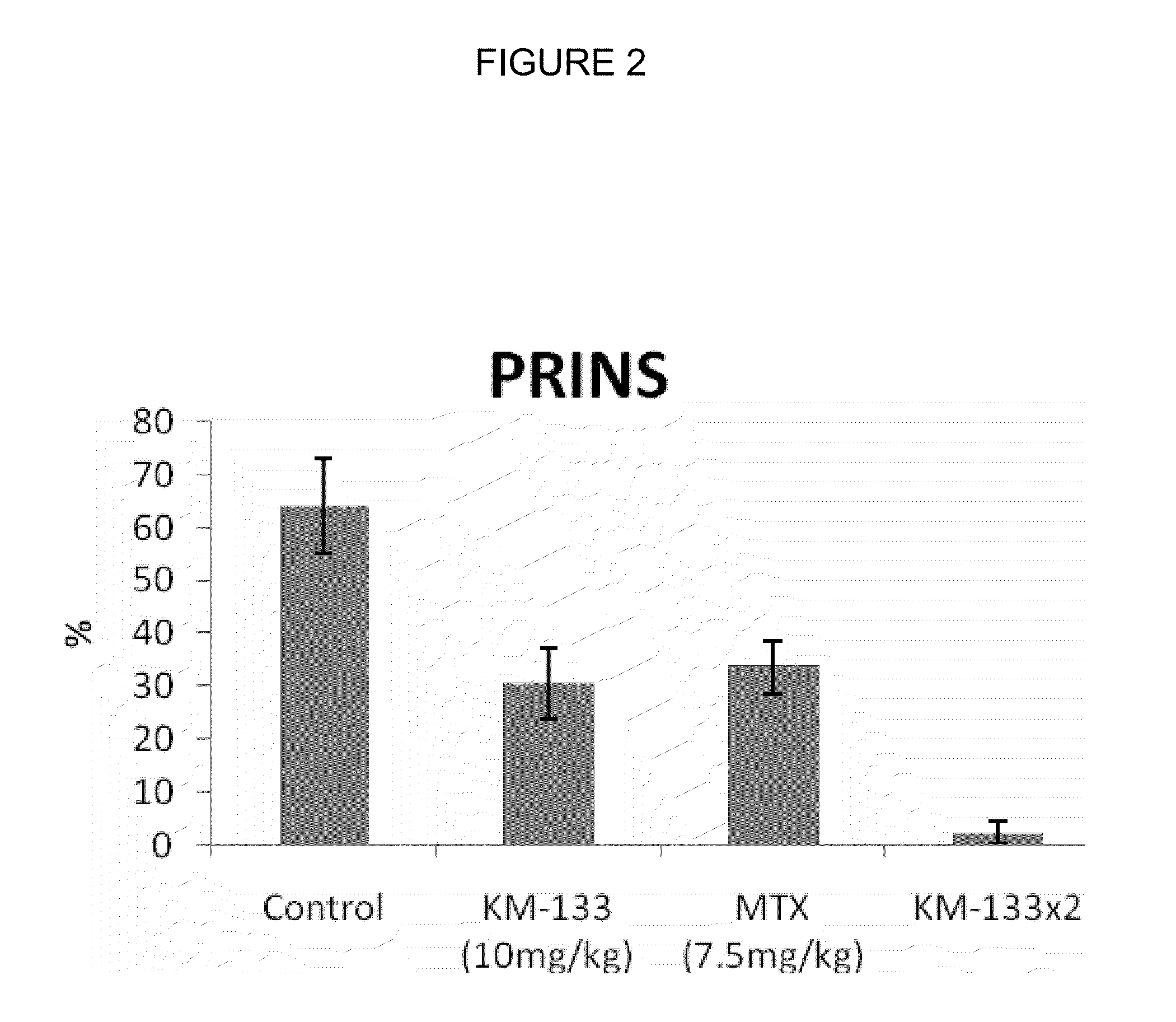
Carbocyclic nucleosides and their pharmaceutical use and compositions
InactiveUS20140200229A1Good metabolic stabilityProlong half-life in vivoBiocideGroup 4/14 element organic compoundsCombinatorial chemistryCarbocyclic nucleoside
Disclosed are compounds of the formula Iand the pharmaceutically acceptable salts of such compounds. Also disclosed are processes for the preparation of such compounds, intermediates used in the preparation of such compounds, and the uses of such compounds in treating inflammatory skin diseases.
Owner:INNOVATION PHARMA INC
Carbocyclic nucleosides and their pharmaceutical use and compositions
InactiveUS8895569B2Good metabolic stabilityProlong half-life in vivoBiocideGroup 4/14 element organic compoundsCombinatorial chemistryCarbocyclic nucleoside
Disclosed are compounds of the formula Iand the pharmaceutically acceptable salts of such compounds. Also disclosed are processes for the preparation of such compounds, intermediates used in the preparation of such compounds, and the uses of such compounds in treating inflammatory skin diseases.
Owner:INNOVATION PHARMA INC
A method for the synthesis of chiral five-membered carbocyclic purine nucleosides based on [3+2] cycloaddition of allenoic acid esters
ActiveCN108558882BHigh stereoselectivitySimple structureOrganic chemistry methodsPtru catalystAlcohol
The invention discloses a method for synthesizing chiral five-membered carbon ring purine nucleoside through [3+2] cycloaddition based on allenoates and belongs to the field of asymmetric synthesis inorganic chemistry. The method takes alpha-purine substituted acrylate 1 and allenoates 2 as raw materials, and chiral STICP as a catalyst; after the raw materials and the catalyst react, chiral five-membered carbon ring nucleoside 3 is obtained; reaction has good enantioselectivity and has moderate to excellent yield. Chiral five-membered carbon ring nucleoside 3 is reduced under the condition ofsodium borohydride to obtain mono-alcohol five-membered carbon ring purine nucleoside 4; then mono-alcohol five-membered carbon ring purine nucleoside 4 is reduced by adopting DIBAL-H to obtain diolfive-membered carbon ring purine nucleoside 5.
Owner:HENAN NORMAL UNIV
A method for synthesizing 2'-spirocyclyl substituted three-membered carbocyclic nucleosides
The invention discloses a method for synthesizing three-membered carbocyclic nucleosides substituted by 2'-spirocyclic groups, belonging to the technical field of organic chemistry. Using α-pyrimidine-substituted acrylate and α-chlorocycloalkanone as raw materials, a series of novel spirocyclic nucleosides were synthesized through the cyclopropanization reaction initiated by the Mike reaction. The invention adopts simple and easy-to-obtain raw materials, and avoids the shortcomings of many steps, low yield and poor universality in the previous methods in the synthesis process. The yield of the spirocyclic pyrimidine nucleoside synthesized by this method is as high as 84%, and the product structure is rich.
Owner:HENAN NORMAL UNIV
Method for synthesizing 2'-spiro-substituted ternary carbocyclic nucleoside
The invention discloses a method for synthesizing 2'-spiro-substituted ternary carbocyclic nucleoside, and belongs to the technical field of organic chemistry. The method for synthesizing the 2'-spiro-substituted ternary carbocyclic nucleoside comprises the steps of using alpha-pyrimidine-substituted acrylate and alpha-chloronaphthenone as raw materials, and synthesizing the spiro nucleoside novelin structure through cyclopropyl cyclization reaction started by Michael reaction. According to the method for synthesizing the 2'-spiro-substituted ternary carbocyclic nucleoside, the adopted raw materials are simple and easy to obtain, in the synthesis process, and the defects of a wide variety of steps, a low yield and poor universality of a former method are overcome. The yield of the spiro pyrimidine nucleoside synthesized through the method is up to 84%, and products are rich in structure.
Owner:HENAN NORMAL UNIV
A group of six-carbocycle nucleoside analogue, its synthesis method and antiviral application
InactiveCN100412083CSaccharide with heterocyclic radicalsOrganic active ingredientsSodium bicarbonate5-fluorocytidine
The disclosed six-membered carbocyclic nucleoside analogues comprise: adenosine analogue, guanosine analogue, carnine analogue, mercaptopurine riboside analogue, cytidine analogue, 5-fluorocytidine analogue, uridine analogue, 5-fluorouridine analogue, and thymidine analogue as well as their acceptable salts formed by equimolar acid in pharmacy. Wherein, the opposite five-step synthesis method using the pinitol, acetone, methane sulfonyl chloride, p-toluenesulfonyl chloride, benzene sulfochloride, and nucleoside base as materials; the pyridine, water, glacial acetic acid, absolute methanol, DMSO, N, N-DMF as the solvent; the p-toluenesulfonic acid, 2, 2-dimethoxylpropane, anhydrous NaSO4, NaHCO3, triethylamine, and anhydrous K2CO3 as the catalyst. This invention restrains specially the replication of HIV and herpesvirus.
Owner:SHANDONG UNIV
Synthetic method of 5-amino-4-carbamyl imidazole ribavirin carbocyclic analog
ActiveCN104140438BFew reaction stepsMild reaction conditionsGroup 5/15 element organic compoundsSynthesis methods5-Formamidoimidazole-4-carboxamide ribotide
The invention discloses a synthesis method of 5-formamidoimidazole-4-carboxamide ribotide analogue. The analysis method comprises the following steps: by taking (3aR,4S,6R,6aS)-methyl-6-amino-2,2-dimethyltetrahydro-3ahydro-cyclo[d][1,3]dioxo-4-carboxylate, triethyl orthoformate and 2-amino-2-cyanoacetamide as raw materials, conducting loop closing through reflux reaction to obtain an intermediate, reducing with lithium borohydride, reacting with phosphorus oxychloride, and then hydrolyzing to obtain the 5-formamidoimidazole-4-carboxamide ribotide analogue. In the mode, the synthesis method of the 5-formamidoimidazole-4-carboxamide ribotide analogue comprises few reaction steps, is mild in reaction conditions, convenient in refining, simple to carry out, high in reaction quality, high in yield, and good in environment friendliness.
Owner:PHARMA SHANGHAI
Carbocyclic nucleoside reverse transcriptase inhibitors
ActiveUS11040975B2Group 5/15 element organic compoundsHeterocyclic compound active ingredientsReverse transcriptaseNucleoside Analog Reverse Transcriptase Inhibitor
The present invention is directed carbocyclic nucleoside reverse transcriptase inhibitors compounds of Formula Iand their use in the inhibition of HIV reverse transcriptase, the prophylaxis of infection by HIV, the treatment of infection by HIV, and the prophylaxis, treatment, and delay in the onset or progression of AIDS and / or ARC.
Owner:MERCK SHARP & DOHME LLC +1
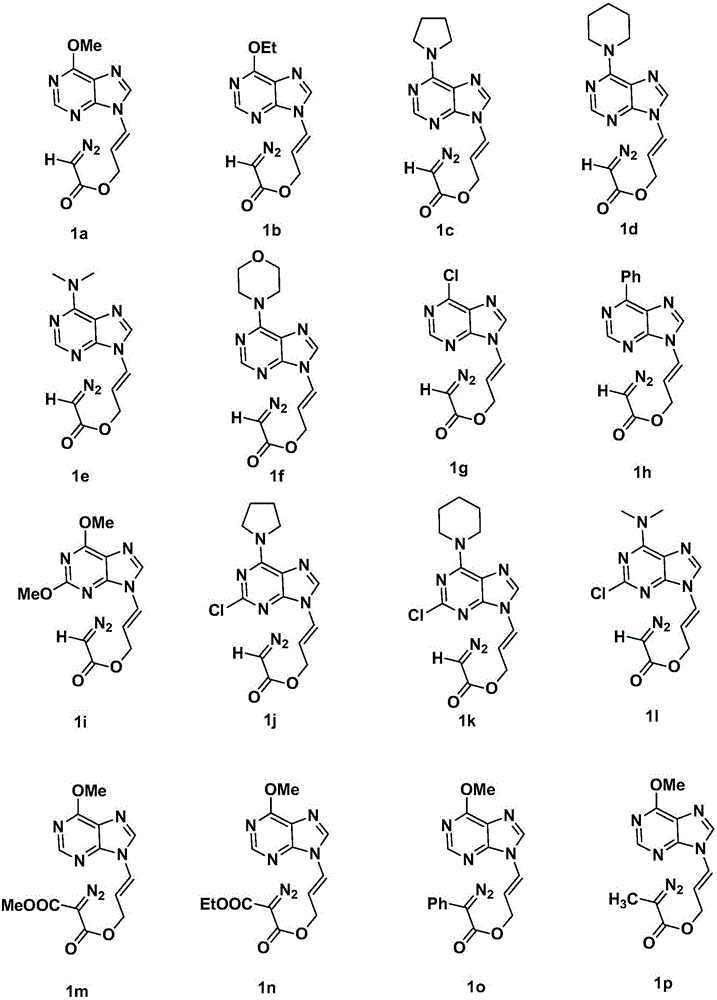
A chiral three-membered carbocyclic pyrimidine nucleoside analog and its preparation method
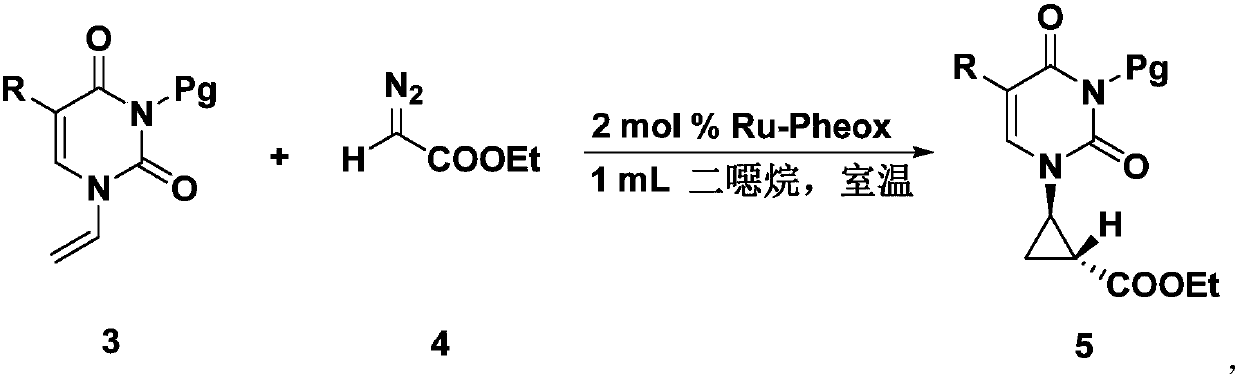
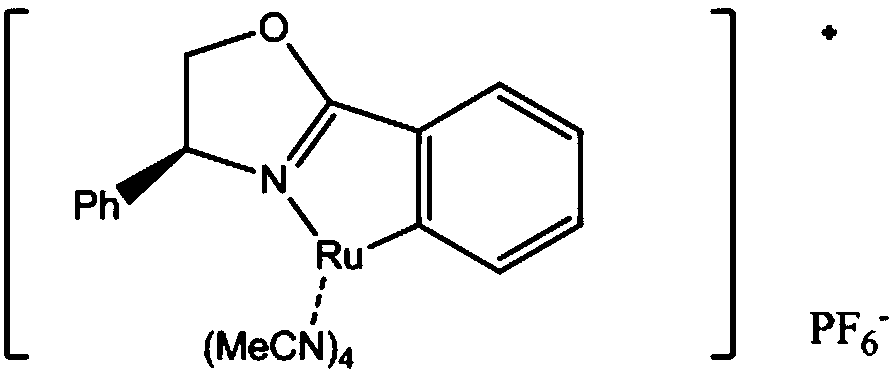
InactiveCN105693627BRaw materials are easy to getEasy to operateOrganic chemistryAntiviral drugRoom temperature
The invention discloses a chiral tri-carbocyclic pyrimidine nucleoside analogue and a preparation method of the chiral tri-carbocyclic pyrimidine nucleoside analogue. The preparation method comprises the following steps of taking dioxane as a solvent, adding Ru-pheox as a ligand and a catalyst, then adding an ethyl diazoacetate reactant, stirring at a room temperature for 4 minutes, and reacting at the room temperature. The preparation method disclosed by the invention is a simple, convenient, green and efficient method for synthesizing the chiral tri-carbocyclic pyrimidine nucleoside analogue, aims to solve the problems of expensive raw materials and complicated process in the process of synthesizing the kind of compound, and has the advantages that a reference value is provided for the synthesis and application of nucleoside drugs, and the raw material is provided for the research of new antiviral drugs and new antitumor drugs.
Owner:HENAN NORMAL UNIV
Structure and Synthesis of Novel Benzyl Phosphoramidate Prodrugs of Nucleoside Compounds
The invention discloses a novel benzyl amido phosphate structure shown as a formula (I). The novel benzyl amido phosphate ester structure can be taken as a prodrug of various nucleoside compounds (including acyclic nucleoside, carbocycle nucleoside, furan ring nucleoside and the like) for enhancing the bioactivity of the nucleoside compounds, so that the novel benzyl amido phosphate ester structure is applied to treatment of virus infection and cancers.
Owner:刘沛
Method for synthesizing five-membered carbocyclic nucleoside
ActiveCN102766173AHigh yieldUse lessSugar derivativesSugar derivatives preparationMarkovnikov's ruleDouble bond
The invention relates to a method for synthesizing five-membered carbocyclic nucleoside, comprising the following steps of: 1) synthesizing a compound 26 in the reaction equation 1, and oxidizing the compound 26 into a dicarbonyl compound 34 by the use of a TEMPO-NaClO system; 2) carrying out selective protection on ketone carbonyl in the compound 34 to generate a compound 35; 3) reducing ketone carbonyl in the compound 35 into a compound 36 with a terminal containing a double bond; 4) performing an addition reaction between the compound 36 and hydrogen bromide by anti-Markovnikov's rule to obtain a bromide 37; and 5) carrying out lithium halogen exchange on the compound 37 to generate nucleophilic addition cyclization within the molecules so as to obtain a compound 38, namely five-membered ring carbasugars; and performing a routine reaction to obtain the five-membered carbocyclic nucleoside. By the adoption of the method provided by the invention, the yield of the five-membered ring carbasugars is greatly raised, and simultaneously usage frequency of extreme low temperature reaction is minimized. All the raw materials used are cheap and easily available.
Owner:SULI CO LTD +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com


![Method for synthesis of chiral five-membered carbocyclic purine nucleoside by asymmetric [3+2] cyclization reaction Method for synthesis of chiral five-membered carbocyclic purine nucleoside by asymmetric [3+2] cyclization reaction](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/b8010604-7fcb-4123-abd5-84739fa69311/FDA0001423608550000011.png)
![Method for synthesis of chiral five-membered carbocyclic purine nucleoside by asymmetric [3+2] cyclization reaction Method for synthesis of chiral five-membered carbocyclic purine nucleoside by asymmetric [3+2] cyclization reaction](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/b8010604-7fcb-4123-abd5-84739fa69311/BDA0001423608560000021.png)
![Method for synthesis of chiral five-membered carbocyclic purine nucleoside by asymmetric [3+2] cyclization reaction Method for synthesis of chiral five-membered carbocyclic purine nucleoside by asymmetric [3+2] cyclization reaction](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/b8010604-7fcb-4123-abd5-84739fa69311/BDA0001423608560000033.png)



![Method for synthesizing chiral pentabasic carbocyclic nucleoside analog by asymmetric [3+2] cycloaddition Method for synthesizing chiral pentabasic carbocyclic nucleoside analog by asymmetric [3+2] cycloaddition](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/e7cb0791-885c-4bfe-87e2-6b4bacd62d4f/BDA0000770668100000023.PNG)
![Method for synthesizing chiral pentabasic carbocyclic nucleoside analog by asymmetric [3+2] cycloaddition Method for synthesizing chiral pentabasic carbocyclic nucleoside analog by asymmetric [3+2] cycloaddition](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/e7cb0791-885c-4bfe-87e2-6b4bacd62d4f/BDA0000770668100000031.PNG)
![Method for synthesizing chiral pentabasic carbocyclic nucleoside analog by asymmetric [3+2] cycloaddition Method for synthesizing chiral pentabasic carbocyclic nucleoside analog by asymmetric [3+2] cycloaddition](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/e7cb0791-885c-4bfe-87e2-6b4bacd62d4f/BDA0000770668100000041.PNG)








![(-)-[gamma]-lactamase, gene, mutant, vector as well as preparation method and application of (-)-[gamma]-lactamase (-)-[gamma]-lactamase, gene, mutant, vector as well as preparation method and application of (-)-[gamma]-lactamase](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/dcdbcdad-834c-4ae5-82a7-74e96b4933f0/BDA0000993124060000061.PNG)
![(-)-[gamma]-lactamase, gene, mutant, vector as well as preparation method and application of (-)-[gamma]-lactamase (-)-[gamma]-lactamase, gene, mutant, vector as well as preparation method and application of (-)-[gamma]-lactamase](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/dcdbcdad-834c-4ae5-82a7-74e96b4933f0/BDA0000993124060000071.PNG)
![(-)-[gamma]-lactamase, gene, mutant, vector as well as preparation method and application of (-)-[gamma]-lactamase (-)-[gamma]-lactamase, gene, mutant, vector as well as preparation method and application of (-)-[gamma]-lactamase](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/dcdbcdad-834c-4ae5-82a7-74e96b4933f0/BDA0000993124060000072.PNG)






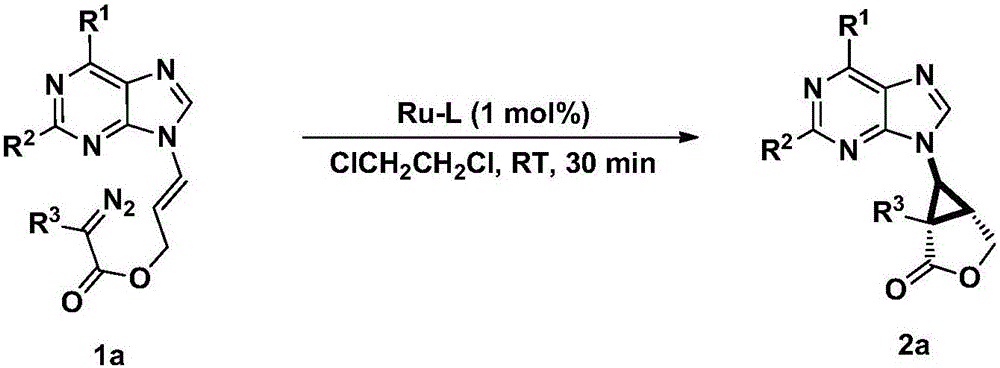









![A method for the synthesis of chiral five-membered carbocyclic purine nucleosides based on [3+2] cycloaddition of allenoic acid esters A method for the synthesis of chiral five-membered carbocyclic purine nucleosides based on [3+2] cycloaddition of allenoic acid esters](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/033f4fe6-12c0-462d-a60c-0f6f10be638f/BDA0001627150010000021.png)
![A method for the synthesis of chiral five-membered carbocyclic purine nucleosides based on [3+2] cycloaddition of allenoic acid esters A method for the synthesis of chiral five-membered carbocyclic purine nucleosides based on [3+2] cycloaddition of allenoic acid esters](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/033f4fe6-12c0-462d-a60c-0f6f10be638f/BDA0001627150010000022.png)
![A method for the synthesis of chiral five-membered carbocyclic purine nucleosides based on [3+2] cycloaddition of allenoic acid esters A method for the synthesis of chiral five-membered carbocyclic purine nucleosides based on [3+2] cycloaddition of allenoic acid esters](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/033f4fe6-12c0-462d-a60c-0f6f10be638f/BDA0001627150010000031.png)