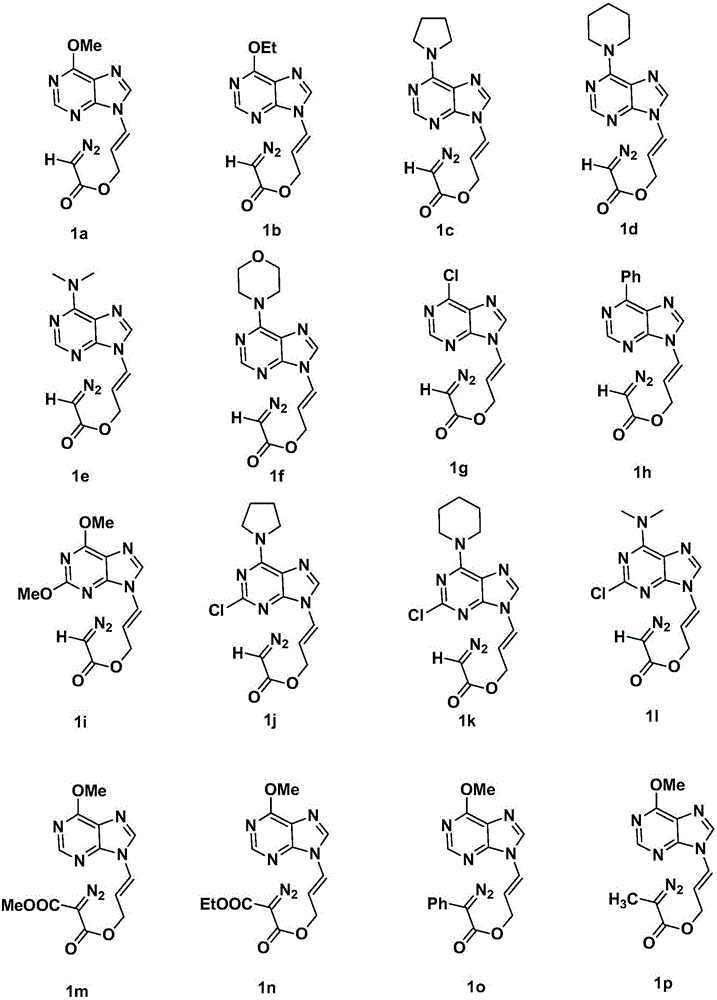
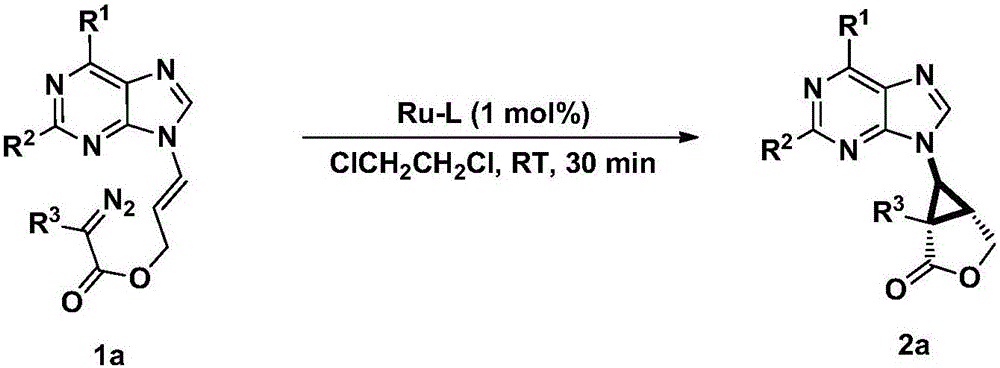Method for synthesizing chiral ternary purine carbocyclic nucleoside analogue through intramolecular asymmetric cycloaddition
A carbocyclic nucleoside, asymmetric technology, applied in the field of intramolecular asymmetric cycloaddition synthesis of chiral three-membered purine carbocyclic nucleoside analogs, can solve the problems of expensive raw materials, complicated processes, etc. The effect of simple operation and cheap and easy-to-obtain catalysts
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023]
[0024] Take a 100mL flask, weigh III (206mg, 1.0mmol), K 2 CO 3 (691mg, 5.0mmol) and bromoacetyl bromide (262μL, 3.0mmol), then add 60mL of dichloromethane solution, react for 1h, quench, extract, and spin dry. Dissolve the obtained intermediate in tetrahydrofuran (50 mL), add N, N'-bis-p-toluenesulfonyl hydrazide, the reaction is lowered to 0 ° C, and DBU (750 μL, 5.0 mmol) is added dropwise. After the reaction is complete, quench , extracted, spin-dried, and column chromatography (PE / EA=1:1) gave a yellow solid. 1 HNMR (400MHz, CDCl 3 ):δ8.50(s,1H),8.08(s,1H),7.26(d,J=14.4Hz,1H),6.65-6.62(m,1H),4.81-4.79(m,2H),4.12( s,3H). 13 CNMR (100MHz, CDCl 3 ): δ161.4, 153.0, 151.3, 139.9, 124.7, 122.2, 115.3, 62.7, 54.5, 46.6. HRMS (ESI): m / zcalcd.forC 11 h 10 N 6 NaO 3 [M+Na] + :297.0707,found297.0713.
Embodiment 2
[0026]
[0027] Take IV (1.53g, 5.0mmol) and p-toluenesulfonyl azide (1.18g, 6.0mmol) into a 100mL flask, and add 50mL of acetonitrile. At 0 °C, DBU (2.24 mL, 15.0 mmol) was added dropwise and monitored by TLC. After the reaction was complete, it was quenched, extracted, spin-dried, and passed through a column (PE / EA=1:1) to obtain a white solid. 1 HNMR (400MHz, CDCl 3 ):δ8.53(s,1H),8.09(s,1H),7.34(d,J=14.4Hz,1H),6.76-6.69(m,1H),4.91(d,J=6.8Hz,2H) ,4.15(s,3H),3.18(s,3H). 13 CNMR (100MHz, CDCl 3 ): δ161.3, 161.0, 153.0, 151.3, 140.0, 125.5, 122.2, 114.4, 63.5, 54.5, 52.8. HRMS (ESI): m / zcalcd.forC 13 h 13 N 6 o 5 [M+H] + :333.0942,found333.0950.
Embodiment 3
[0029]
[0030] Take a 100mL flask, weigh V (3.38g, 10.0mmol), TsNHNH 2 (1.86g, 10.0mmol) was dissolved in 50mL toluene, the reaction was refluxed for 9h, spin-dried, then dissolved in dichloromethane, the reaction was transferred to 0°C and Et 3 N (1.02g, 10.0mmol), after the completion of the reaction was detected by TCL, extracted, spin-dried, and passed through the column (PE / EA=1:1) to obtain a yellow solid. 1 HNMR (400MHz, CDCl 3 ):δ8.60(s,1H),8.11(s,1H),7.50-7.32(m,5H),7.20(t,J=7.2Hz,1H),6.79-6.72(m,1H),4.99( d,J=6.8Hz,2H),4.20(s,3H). 13 CNMR (150MHz, CDCl 3 ): δ161.4, 153.0, 151.3, 139.9, 129.2, 126.2, 125.3, 125.1, 124.2, 122.2, 115.2, 62.9, 54.5. HRMS (ESI): m / zcalcd.forC 17 h 15 N 6 o 3 [M+H] + :351.1200,found351.1208.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com