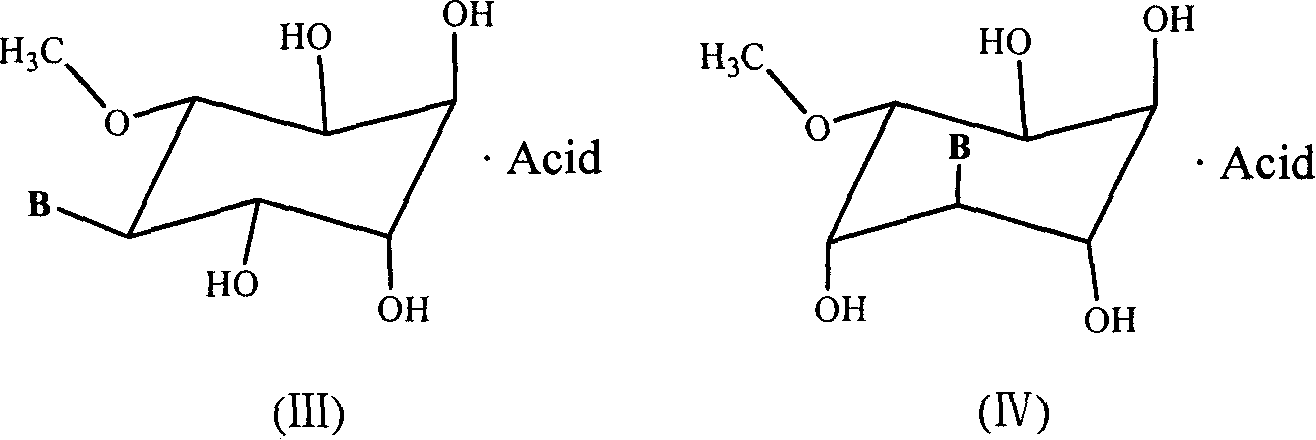
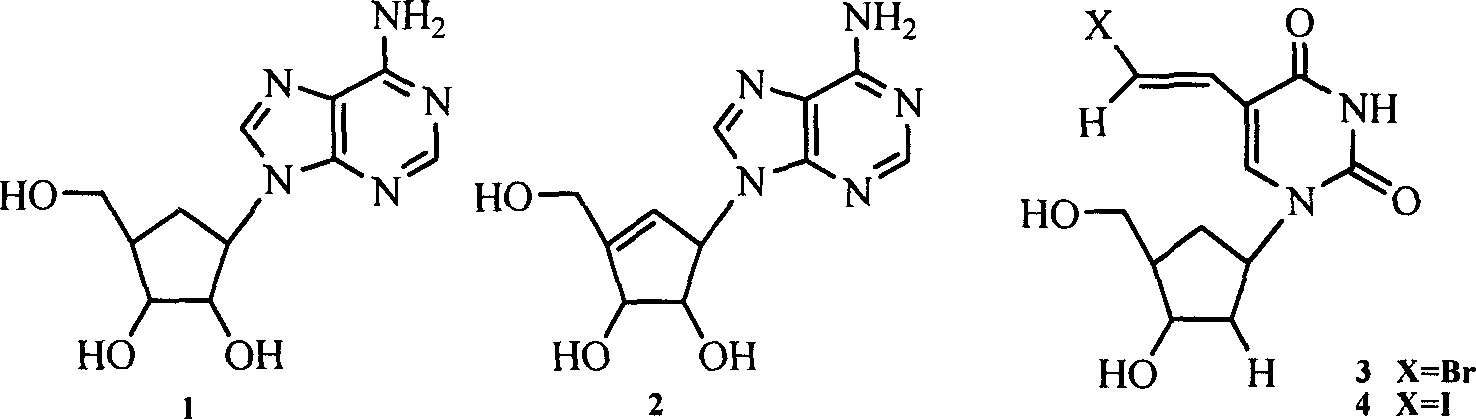
A group of six-carbocycle nucleoside analogue, its synthesis method and antiviral application
A carbocyclic nucleoside and analog technology, applied in the field of six-membered carbocyclic nucleoside analogs, can solve problems such as no new compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Example 1: 3-O-methyl-D-inositol-5-(9'-adenine) nucleoside and 3-O-methyl-D-inositol-4-(9'-adenine) nucleoside Synthesis of glycosides (compound numbers are 1a and 1b respectively)
[0056] (1) Synthesis of 1,2:5,6-di-O-isopropylidene-3-O-methyl-D-inositol
[0057] After adding 25mLDMF, 50mL 2,2-di-methoxypropane and 150mL acetone in a 500mL three-necked flask, add 10.0g (51.5mmol) of 3-O-methyl-D-inositol, 380mg of p-toluenesulfonic acid ( 2.0mmol) and anhydrous magnesium sulfate 9.0g, the suspension was stirred at room temperature for 16 hours, 1.0g of sodium bicarbonate was added, stirred for half an hour and then filtered, the filter residue was washed 3 times with ethyl acetate and combined with the filtrate, concentrated under reduced pressure Recrystallize with a mixed solvent of n-hexane and ether in equal proportions to obtain 12.6 g (46.0 mmol, 89%), the crude product can be directly used in the next step reaction. Melting point: 95-96°C, [α] D 20 -41.5° ...
Embodiment 2
[0068] Example 2: 3-O-methyl-D-inositol-5-(1'-cytosine) nucleoside and 3-O-methyl-D-inositol-4-(1'-cytosine) nucleoside Synthesis of glycosides (compound numbers are 2a and 2b respectively)
[0069] (1) Synthesis of 1,2:5,6-di-O-isopropylidene-3-O-methyl-D-inositol
[0070] Synthesized as in Example 1 (1) method and conditions.
[0071] (2) Synthesis of 1,2:5,6-di-O-isopropylidene-3-O-methyl-4-methanesulfonyl-D-inositol
[0072] Synthesize as embodiment 1 (2) method and condition.
[0073] (3) Synthesis of 3-O-methyl-4-methanesulfonyl-D-inositol
[0074] Synthesize as embodiment 1 (3) method and condition.
[0075] (4) Synthesis of 3-O-methyl-4,5-epoxy-D-inositol
[0076] Synthesized as in Example 1(4) method and conditions.
[0077] (5) 3-O-methyl-D-inositol-5-(1'-cytosine) nucleoside and 3-O-methyl-D-inositol-4-(1'-cytosine) nucleoside (compound numbers are respectively 2a and 2b) synthetic
[0078] Add 352mg (2mmol) of epoxy compound and 333mg (3mmol) of dry cytosine ...
Embodiment 3
[0082] Example 3: Synthesis of 3-O-methyl-D-inositol-5-(9'-adenine) nucleoside fumaric acid complex (1c)
[0083] The synthetic method of 3-O-methyl-D-inositol-5-(9'-adenine) nucleoside fumaric acid complex (1c) is as follows: 3-O-methyl-D-inositol-5- (9'-Adenine) nucleoside 311mg (1mmol) mixed with fumaric acid 124mg (1mmol) and 2ml of isopropanol, the resulting mixture was stirred at 50 ° C until the solid was completely dissolved, then cooled at room temperature to precipitate crystals, filtered to obtain a solid , and the product was washed with ether, dried in vacuo to obtain 318 mg, the yield was 73%, except for 3-O-methyl-D-inositol-5-(9'-adenine) nucleoside signal in 1H NMR (DMSO), The signal of fumaric acid double bond hydrogen was shown at δ (ppm) 6.216 (s, 2H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com