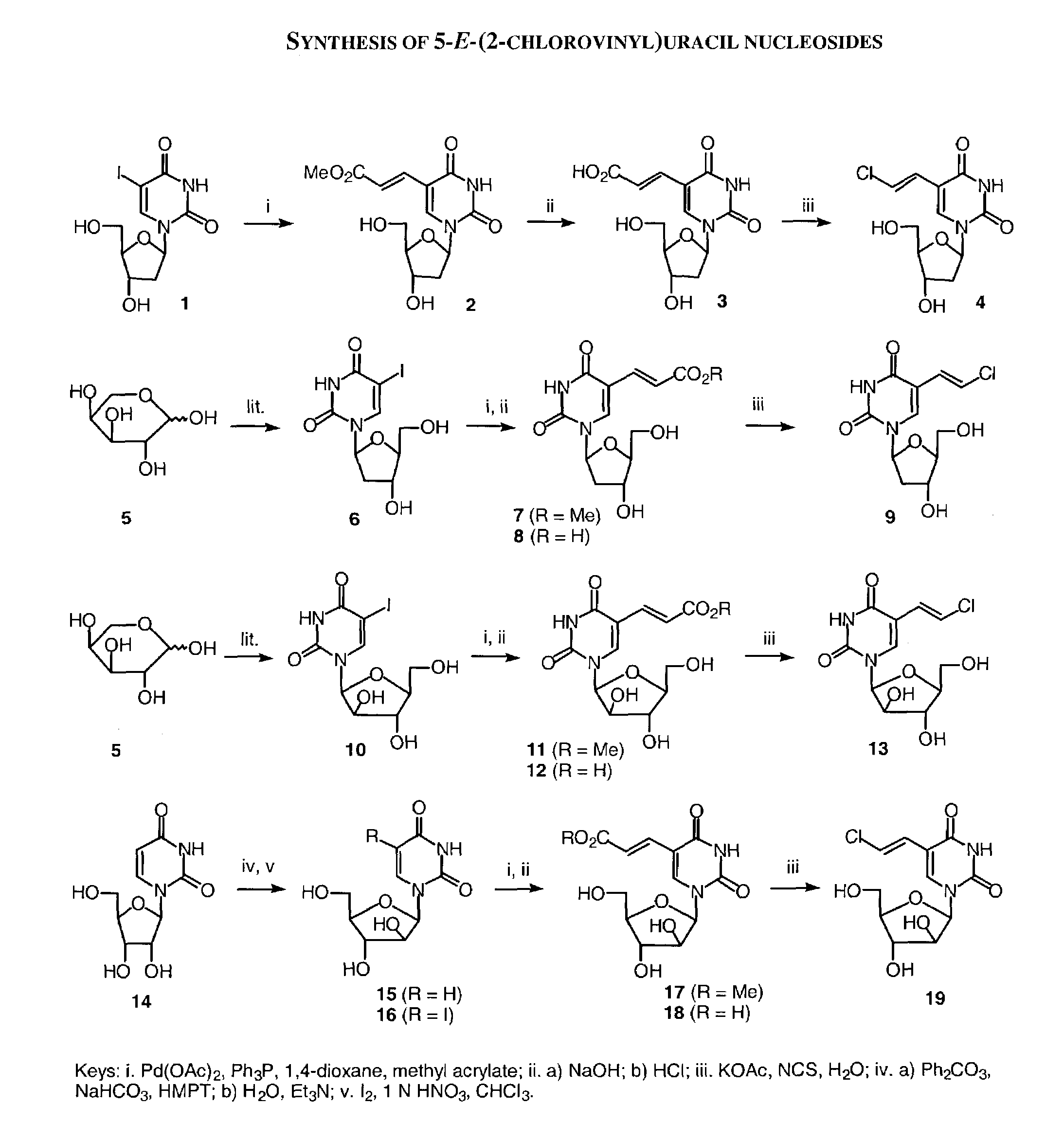
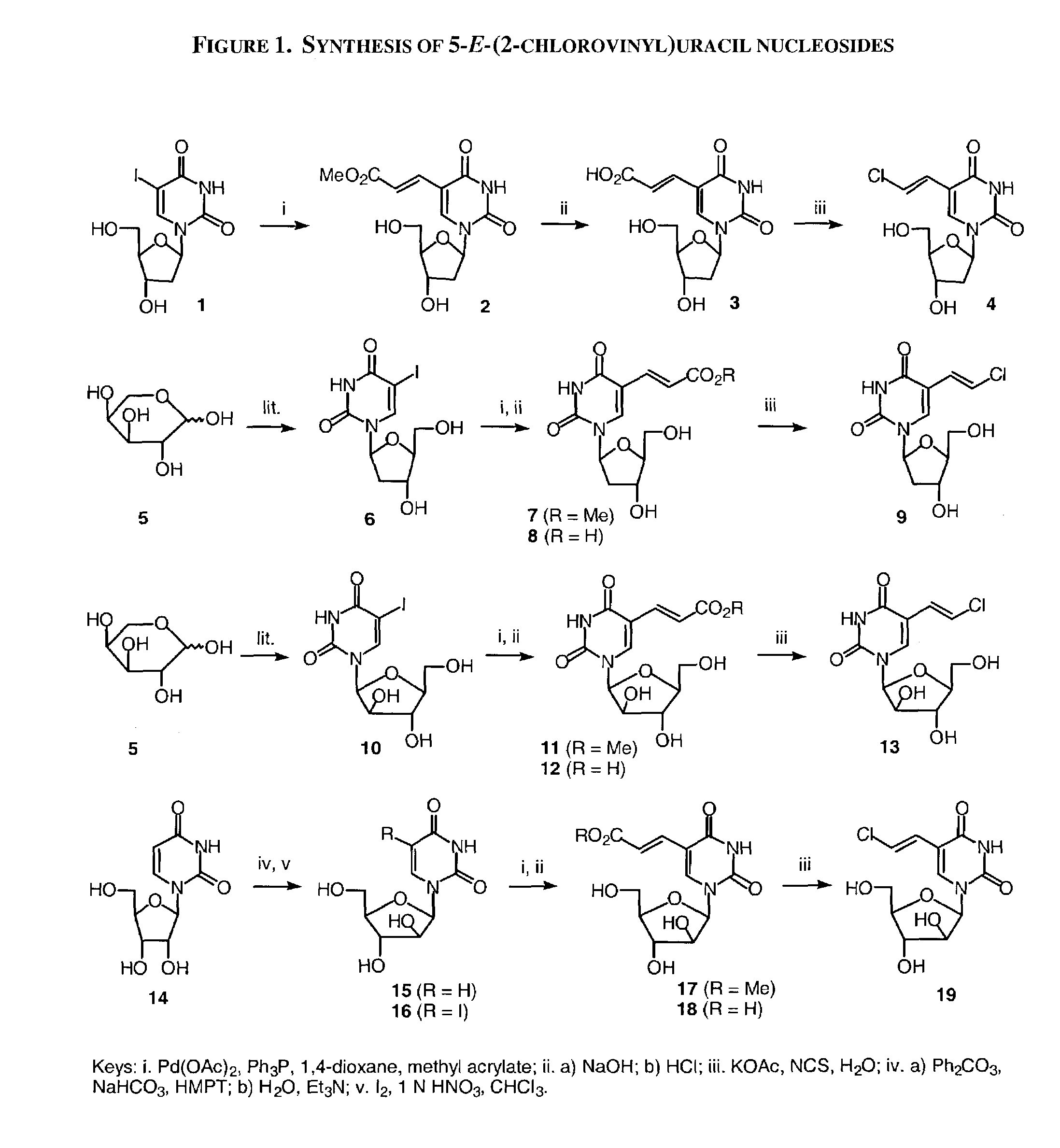
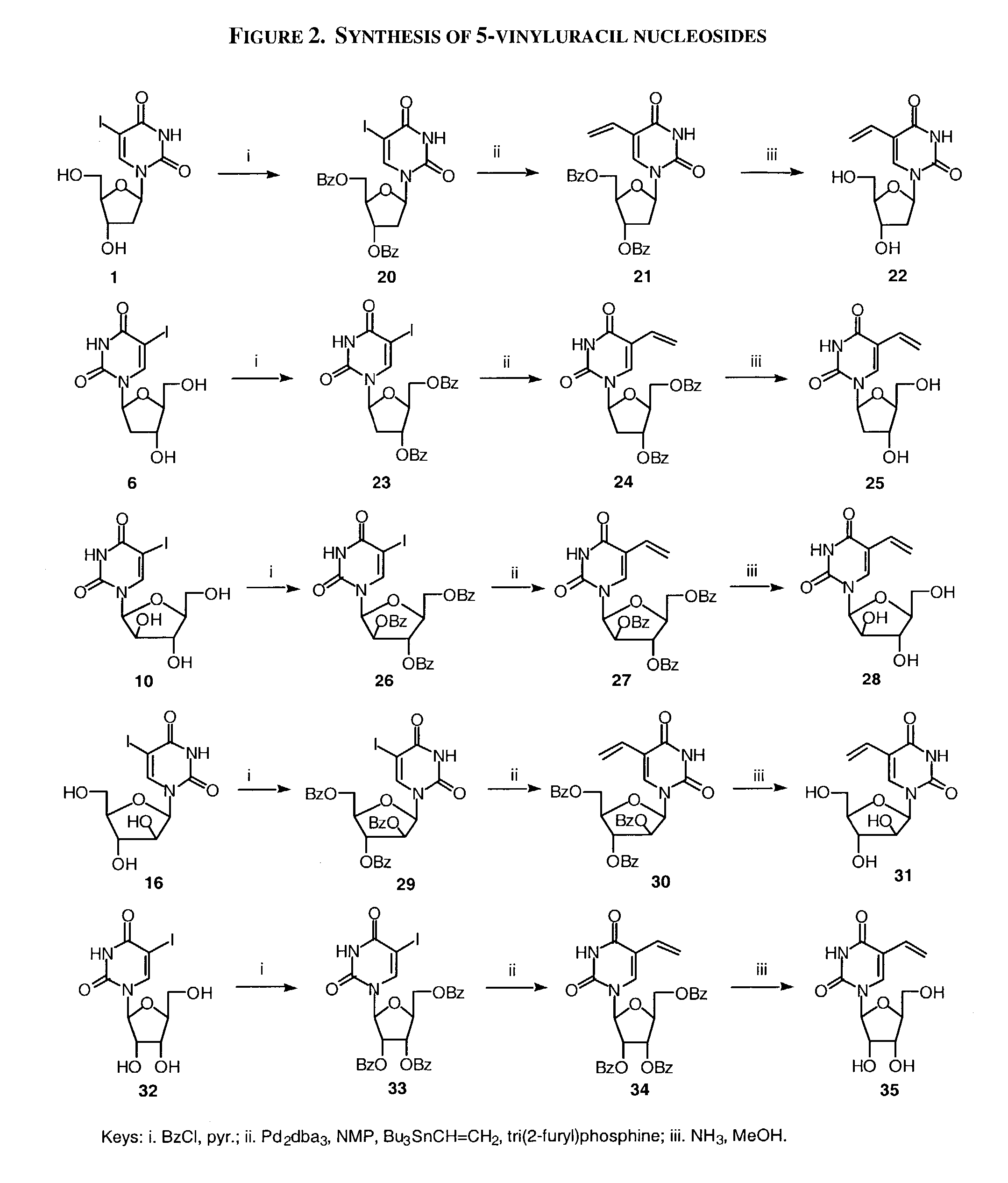
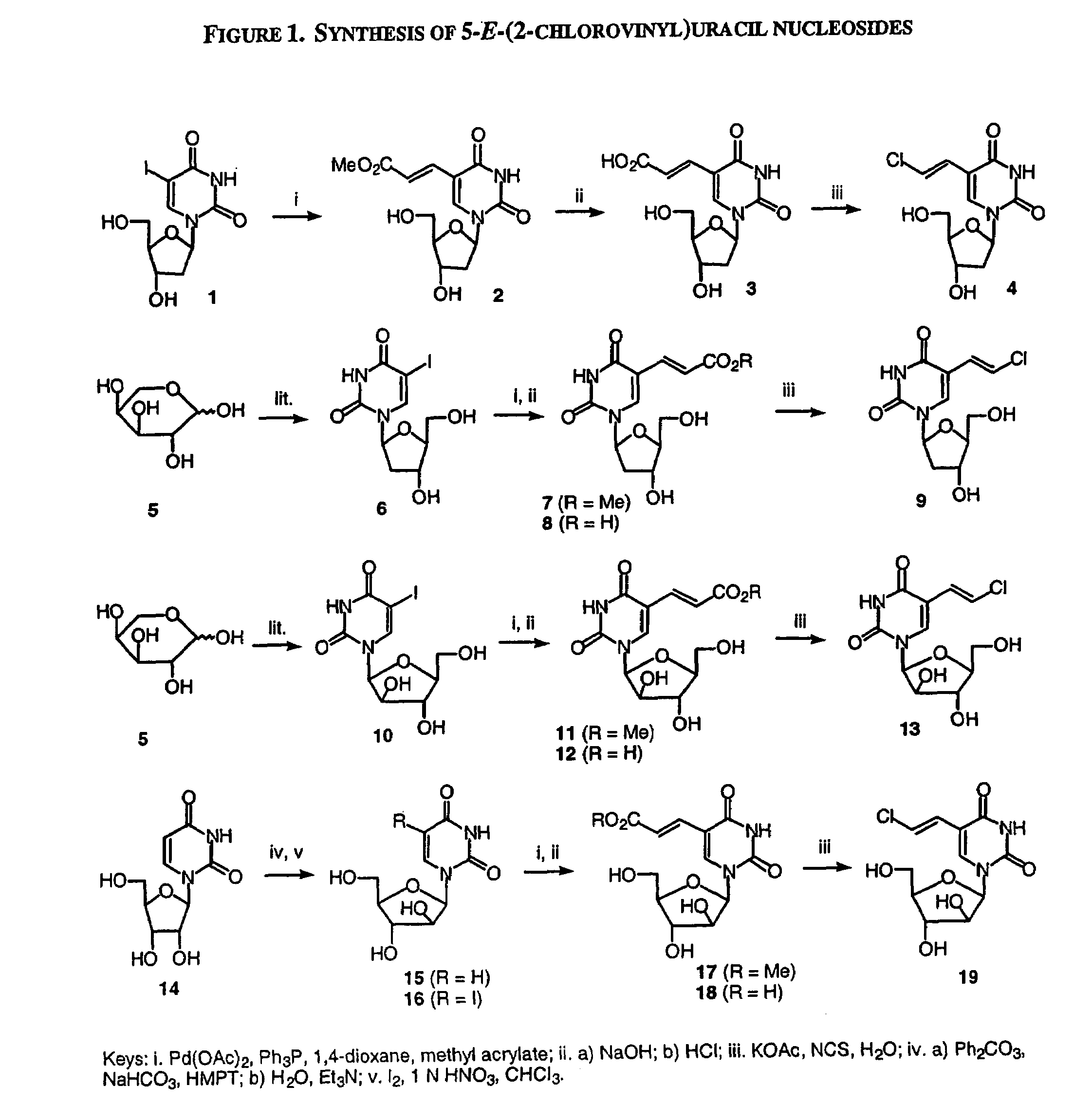
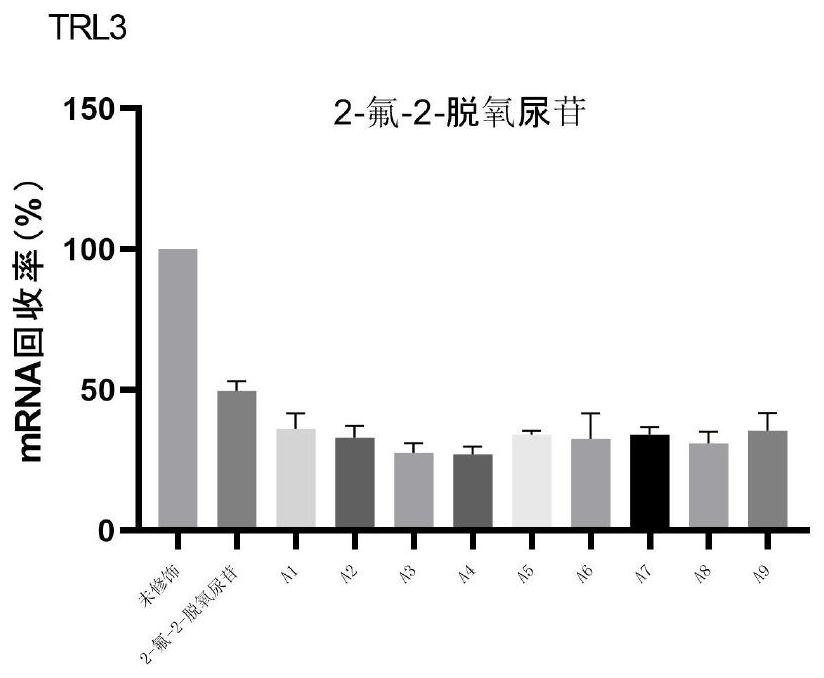
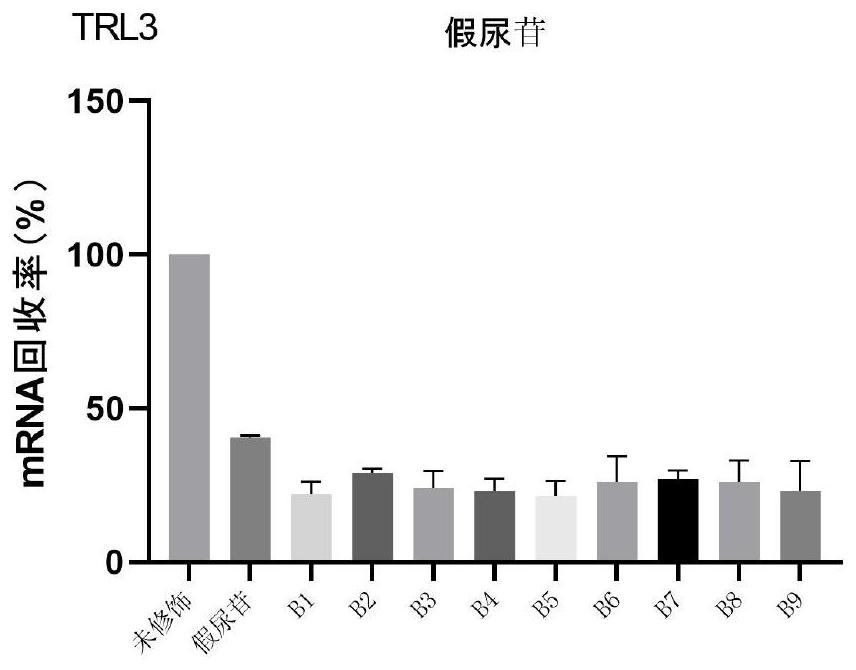
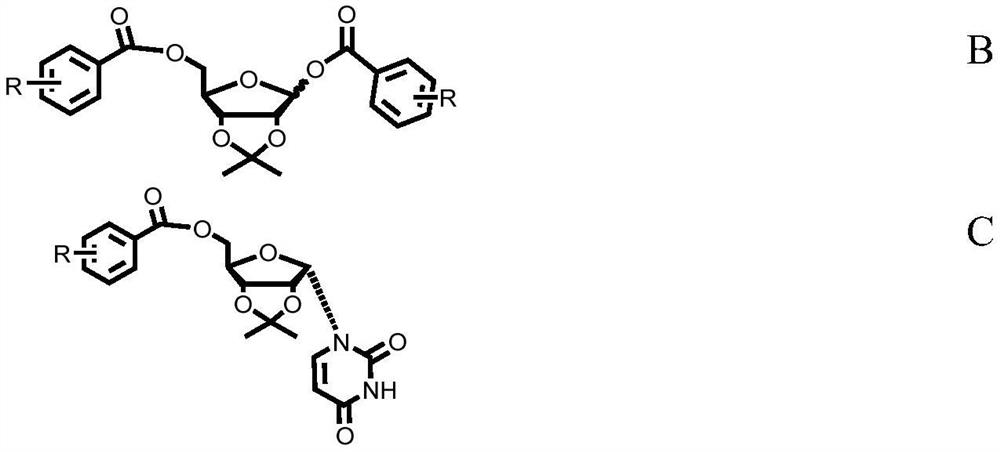
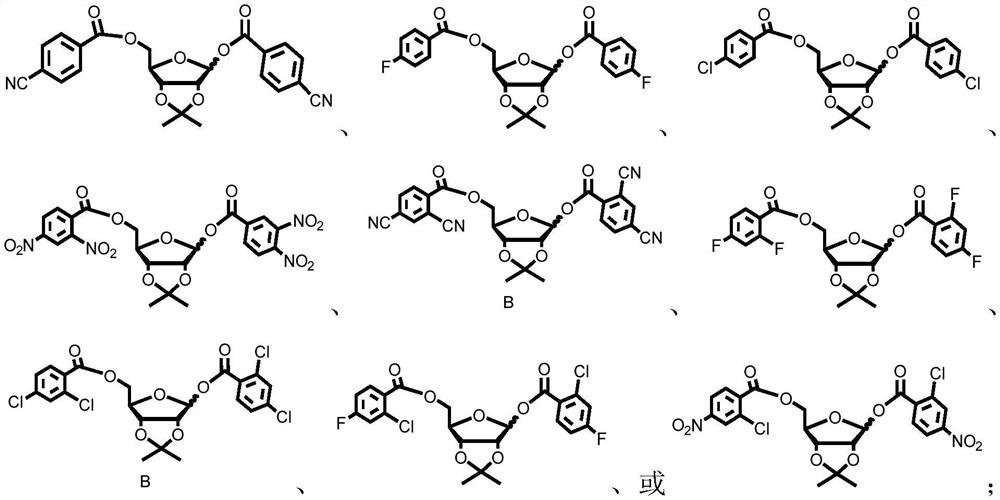
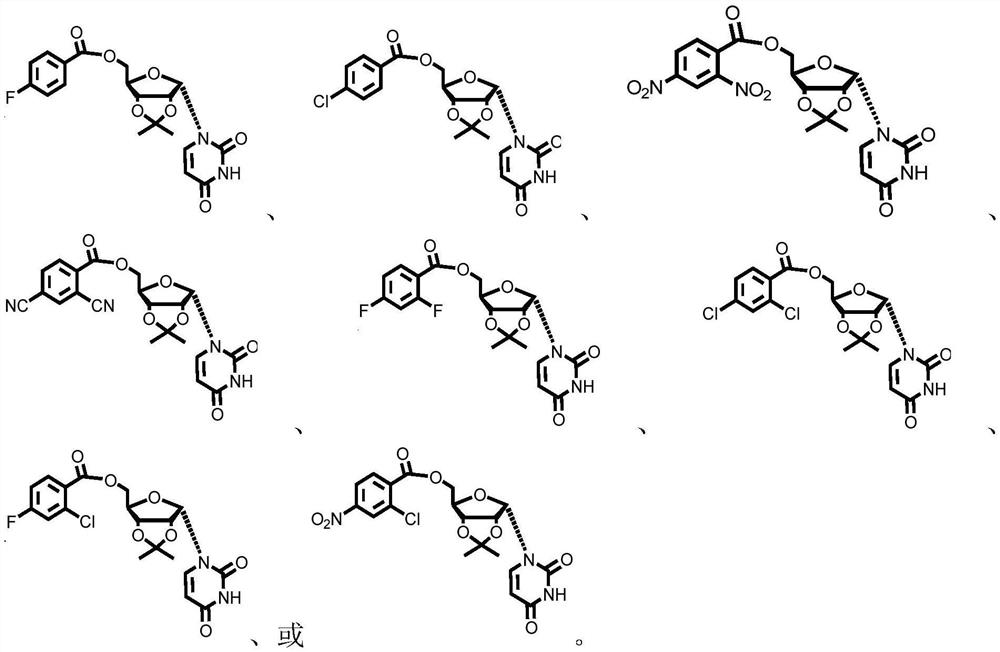
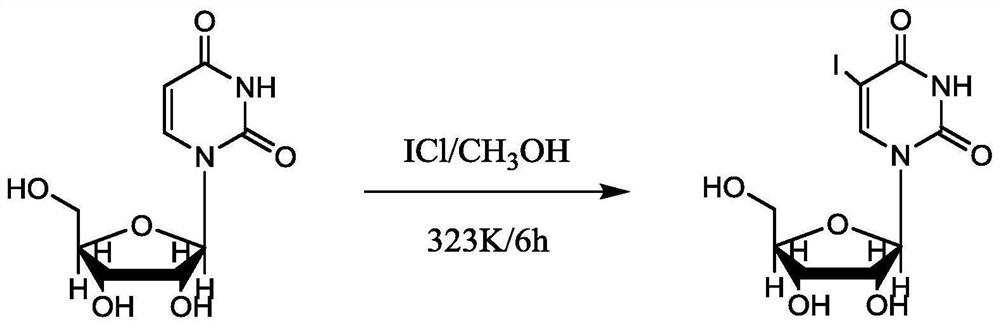
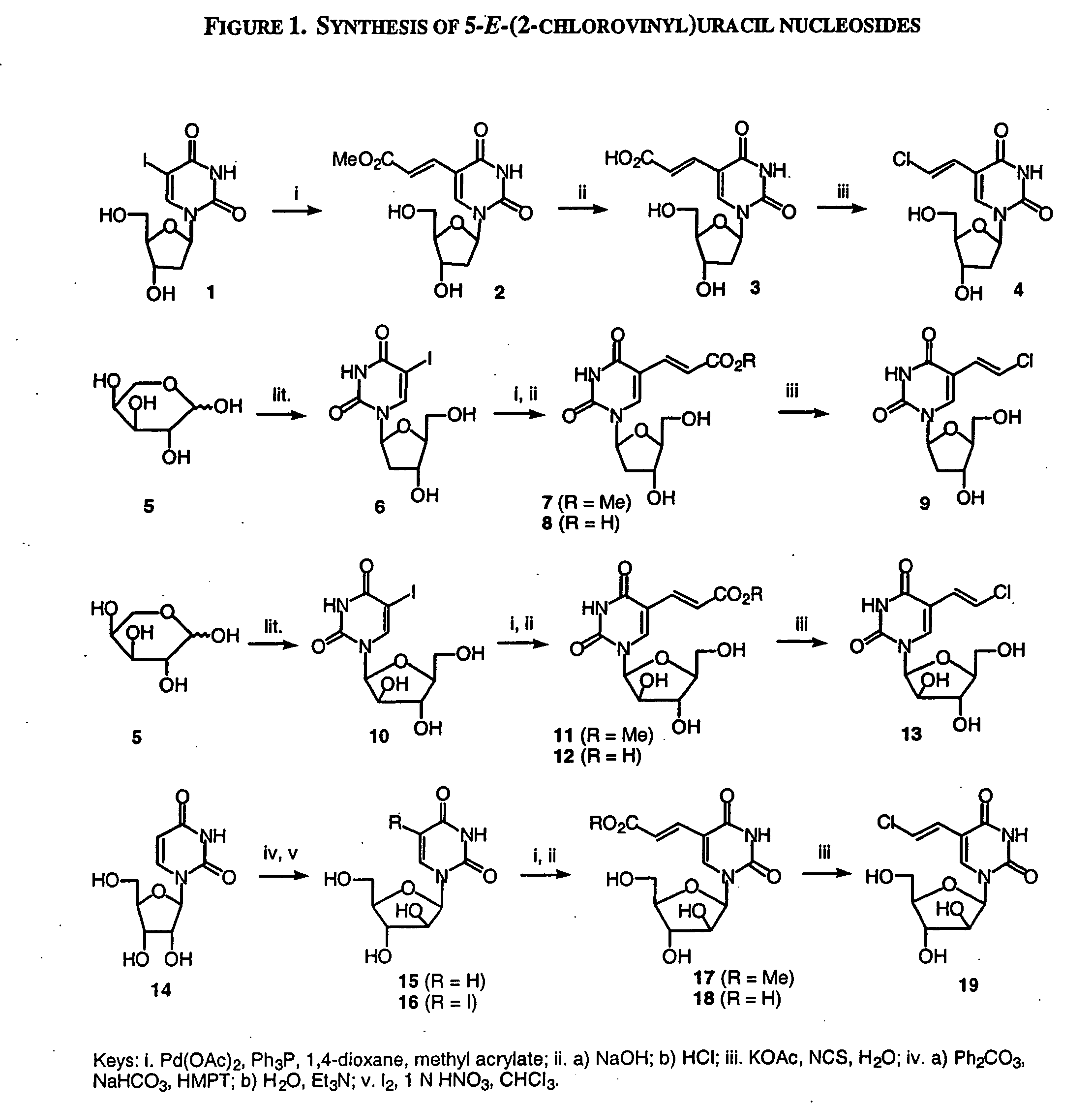
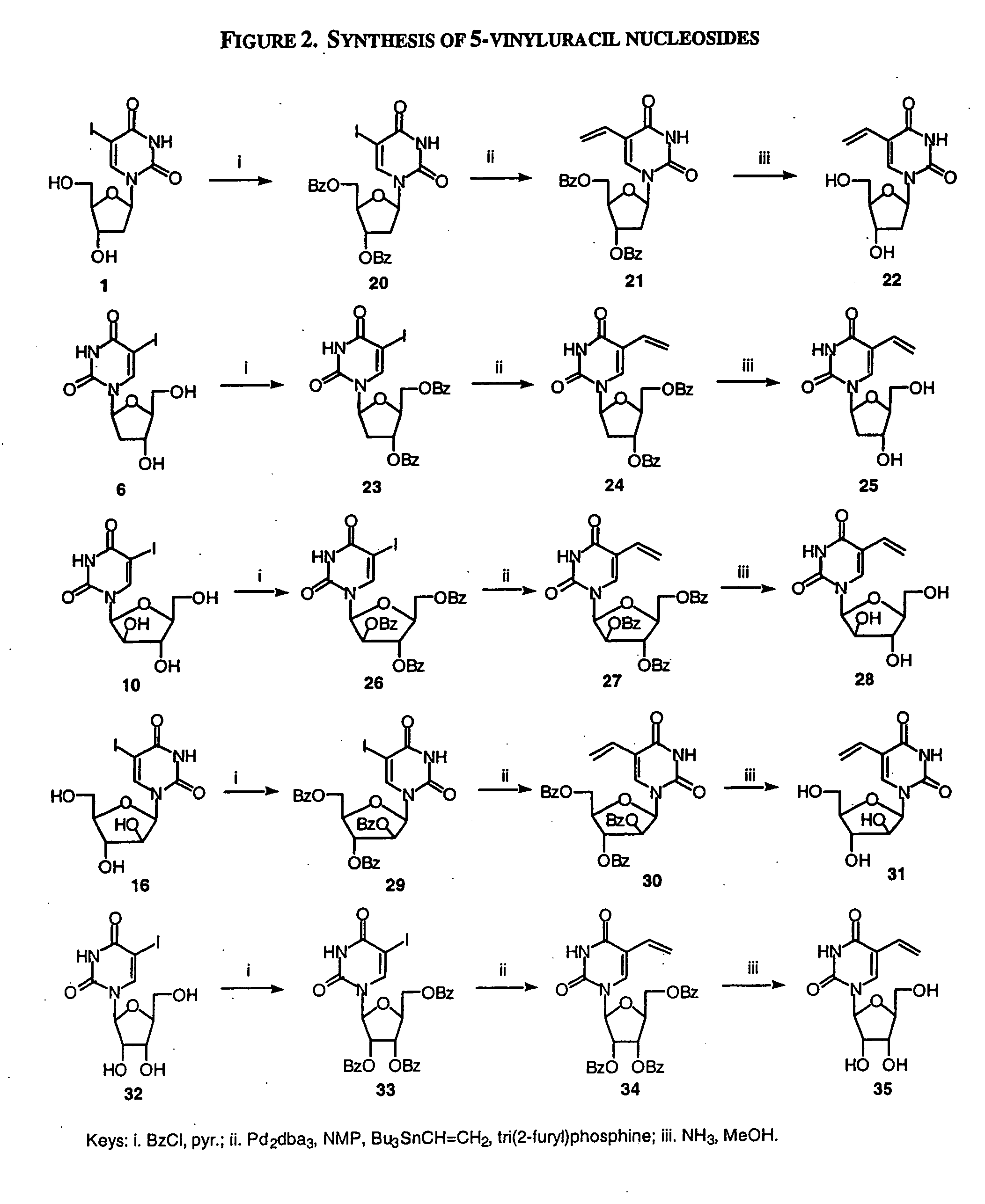
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
35 results about "Uracil nucleoside" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
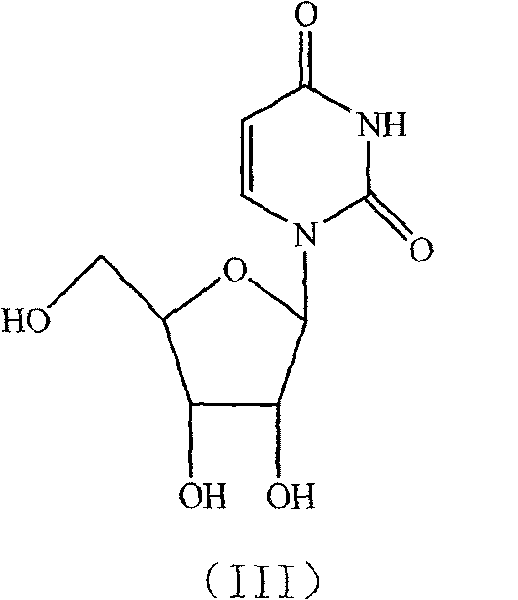
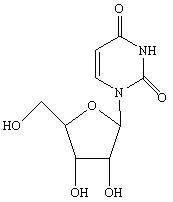
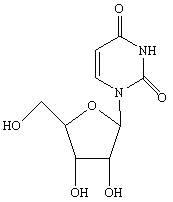
Condensation product of a uracil base plus a sugar.
Treatment of EBV and KHSV infection
InactiveUS7211570B2Growth inhibitionIncreases activity and bioavailability and stabilityBiocideGenetic material ingredientsMedicineDiluent
A method and composition for the treatment, prevention and / or prophylaxis of a host, and in particular, a human, infected with Epstein-Barr virus (EBV), is provided that includes administering an effective amount of a 5-substituted uracil nucleoside or its pharmaceutically acceptable salt or prodrug, optionally in a pharmaceutically acceptable diluent or excipient.
Owner:EMORY UNIVERSITY +1
Treatment of EBV and KHSV infection and associated abnormal cellular proliferation
InactiveUS7638502B2Increases activity and bioavailability and stabilityEffective destructionBiocidePeptide/protein ingredientsMedicineDiluent
A method and composition for the treatment, prevention and / or prophylaxis of a host, and in particular, a human, infected with Epstein-Barr virus (EBV), is provided that includes administering an effective amount of a 5-substituted uracil nucleoside or its pharmaceutically acceptable salt or prodrug, optionally in a pharmaceutically acceptable diluent or excipient.
Owner:GILEAD PHARMASSET LLC
Beta-2'-deoxygenation-ramification of nucleotide, synthetic method and application of medication
InactiveCN1626543AHigh anti-HBV activityHigh purityOrganic active ingredientsSugar derivativesDiseaseMedicine
A beta-L-2'-deoxyl-nucleoside derivative used for treating the diseases associated with HBV and HIV is prepared from beta-L-2'-deoxy-uracil nucleoside through etherifying, sulfurizing, and reducing. Its advantages are high curative activity and low toxic by-effect.
Owner:QUALITY TEST & ANALYTIC MEASUREMENT RES CENT HENAN ACAD OF SCI
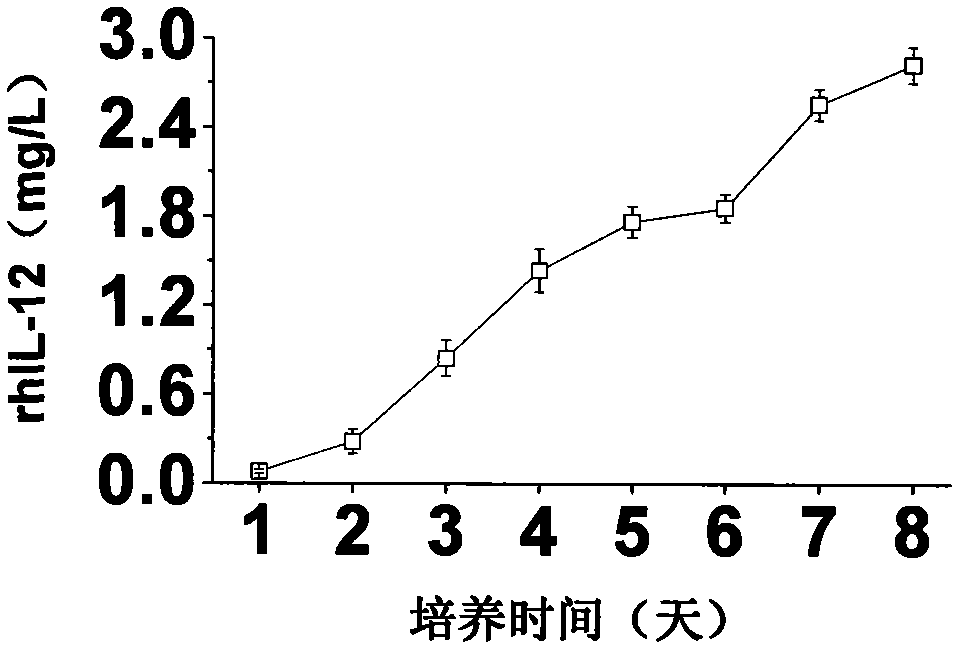
A large-scale serum-free culture method of rhil-12 engineered cells
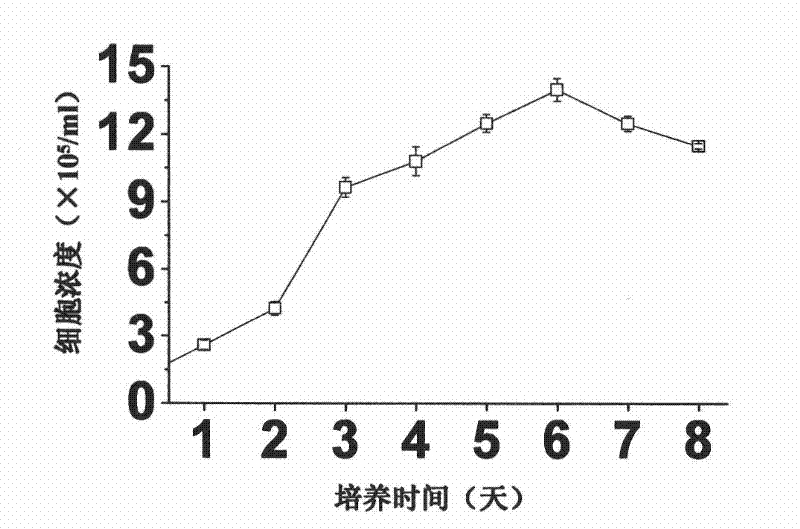
The invention relates to a large-scale serum-free culture method for rhIL-12 engineering cells, which comprises the following step of: inoculating rhIL-12 engineering cells in a logarithmic phase into a serum-free and protein-free medium for culture, wherein the medium is a CD CHO liquid medium comprising sodium pyruvate at the final concentration of 0.8 to 1.2mM, hypoxanthine at the final concentration of 0.075 to 0.125mM, thymidine at the final concentration of 0.012 to 0.020mM, adenosine at the final concentration of 0.5 to 0.9mg / L, guanosine at the final concentration of 0.5 to 0.9mg / L, cytidine at the final concentration of 0.5 to 0.9mg / L, uridine at the final concentration of 0.5 to 0.9mg / L, L-glutamine at the final concentration of 0.4 to 0.8mg / L, L-asparagine at the final concentration of 0.4 to 0.8mg / L, L-proline at the final concentration of 1.5 to 2.0mg / L and non-essential amino acid at the final concentration of 0.08 to 0.125mM. The invention also provides a medium used in the method. By the medium and the culture method, a high-yield and high-activity recombinant human interleukin-12 can be obtained.
Owner:UNIV OF SCI & TECH OF CHINA
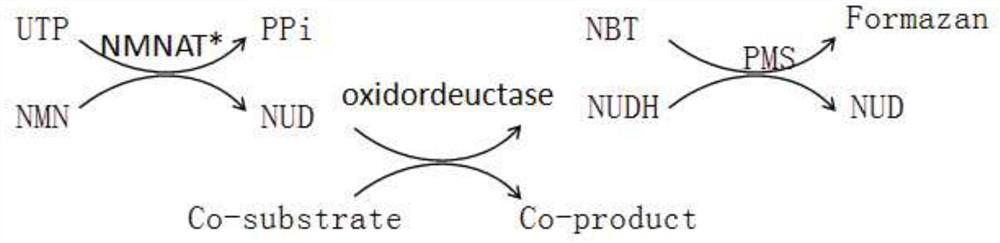
Method for enzymatic synthesis of nicotinamide uracil dinucleotide
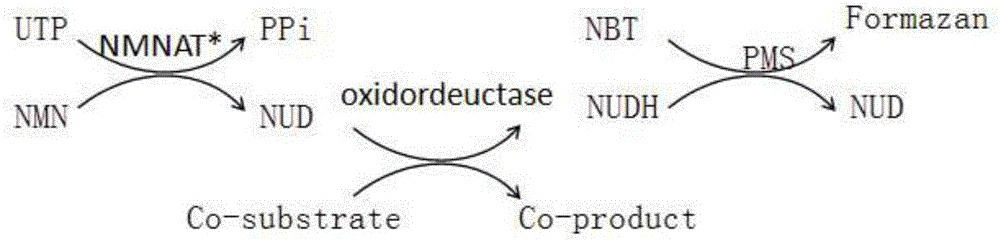
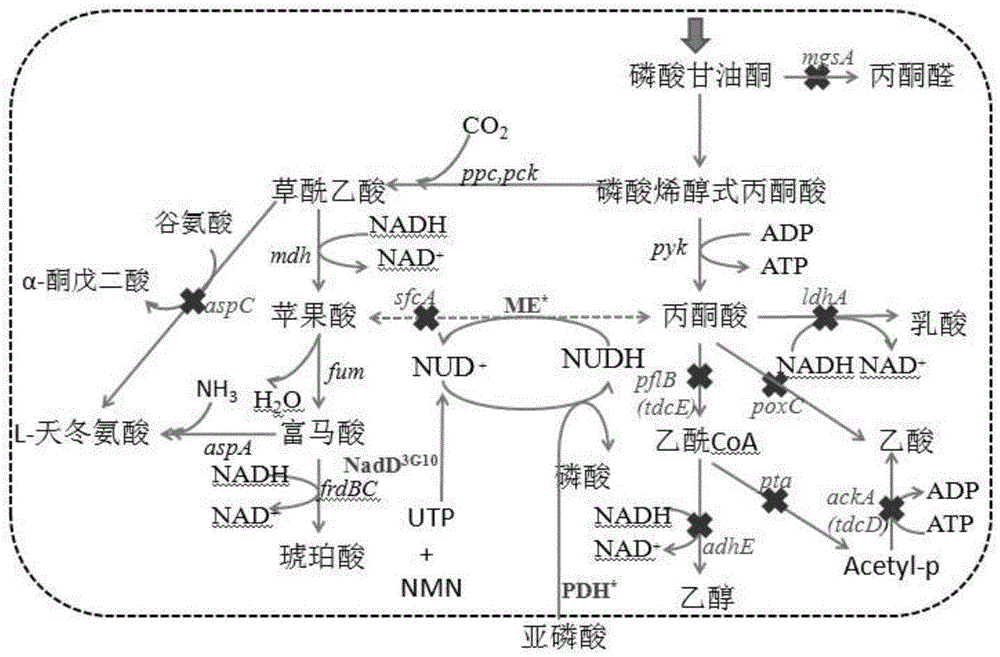
The invention relates to a method for enzymatic synthesis of nicotinamide uracil dinucleotide and an application of the method. The nicotinamide uracil dinucleotide is prepared by catalyzing a coupling reaction between nicotinamide mononucleotide and uracil nucleoside triphosphate with a nicotinamide mononucleotide adenylyltransferase mutant serving as a catalyst. A coding gene of the nicotinamide mononucleotide adenylyltransferase mutant is expressed in microorganism cells; the nicotinamide uracil dinucleotide is synthesized by engineering bacteria on the basis of an endogenous metabolite; and the intracellular nicotinamide uracil dinucleotide, as a coenzyme, can selectively mediate an oxidation-reduction reaction, so that the yield of a target metabolite is improved. According to a typical embodiment, a succinic acid yield is improved by 35%.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
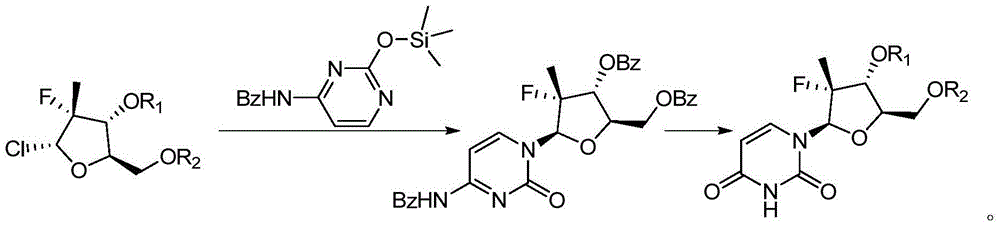
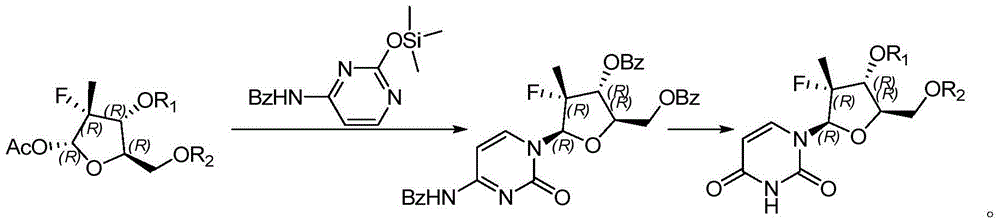
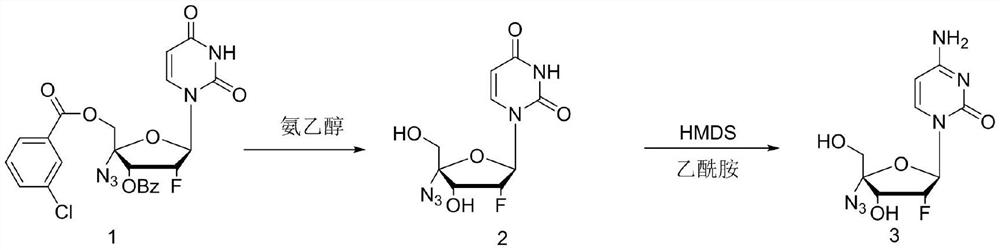
Preparation method for uracil fluoride nucleoside analogue
InactiveCN105153256AEasy to controlShort reaction pathSugar derivativesSugar derivatives preparationArylTrimethylsilyl
The invention discloses a preparation method for uracil fluoride nucleoside analogue comprising reaction b or reaction a to reaction b in the synthesis route shown in the description. R' and R'' are independently selected from trimethylsilyl group, tertbutyldiphenylsilyl group, TBS group or triethylsilyl group; R1 and R2 are independently selected from H, C1-C10 alkyl group, C1-C10 alkylsulfonyl group or aryl-substituted C1-C10 alkylsulfonyl group, wherein the aryl group is phenyl group, biphenylyl group or naphthyl group; A is selected from chlorine, bromine, iodine, hydroxyl group, OC(=O)R3, OS(=O)2OR3, wherein R3 is selected from C1-C5 alkyl group. The preparation method has short reaction route, simple operation, high reaction yield, low cost, is easy to control the reaction condition, has cheap and easily available used raw materials and reagents, has no special requirement for the device, and is suitable for large scale production.
Owner:SHANGHAI ACEBRIGHT PHARMA GRP
A group of six-carbocycle nucleoside analogue, its synthesis method and antiviral application
InactiveCN1803819ASaccharide with heterocyclic radicalsOrganic active ingredientsSodium bicarbonate5-fluorocytidine
The disclosed six-membered carbocyclic nucleoside analogues comprise: adenosine analogue, guanosine analogue, carnine analogue, mercaptopurine riboside analogue, cytidine analogue, 5-fluorocytidine analogue, uridine analogue, 5-fluorouridine analogue, and thymidine analogue as well as their acceptable salts formed by equimolar acid in pharmacy. Wherein, the opposite five-step synthesis method using the pinitol, acetone, methane sulfonyl chloride, p-toluenesulfonyl chloride, benzene sulfochloride, and nucleoside base as materials; the pyridine, water, glacial acetic acid, absolute methanol, DMSO, N, N-DMF as the solvent; the p-toluenesulfonic acid, 2, 2-dimethoxylpropane, anhydrous NaSO4, NaHCO3, triethylamine, and anhydrous K2CO3 as the catalyst. This invention restrains specially the replication of HIV and herpesvirus.
Owner:SHANDONG UNIV
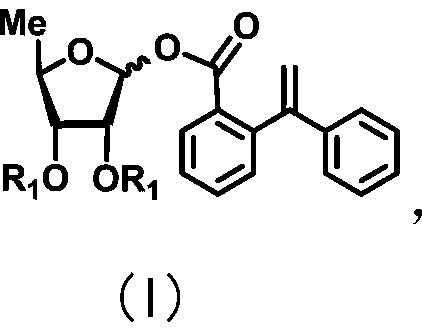
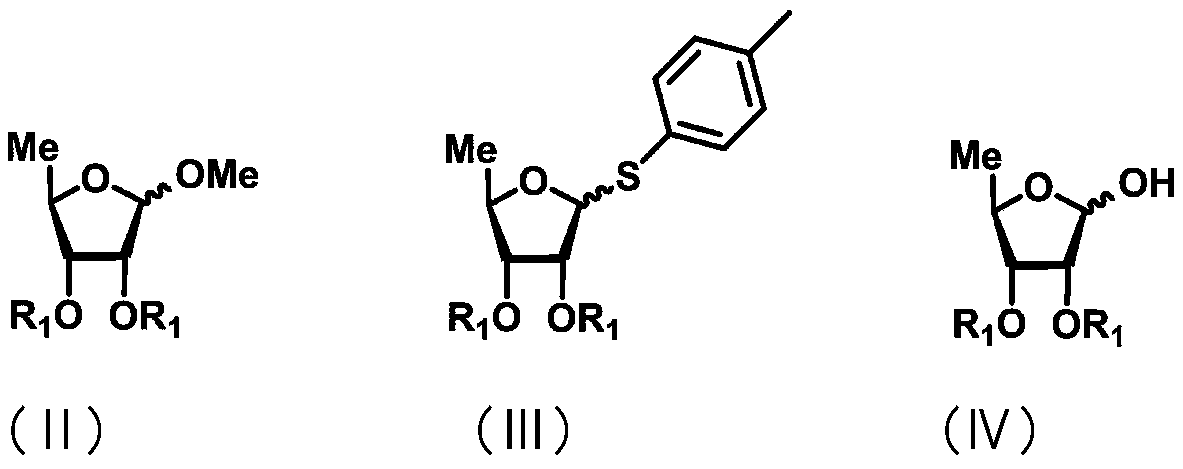
Uracil nucleoside derivative and method for preparing doxifluridine medicine by using uracil nucleoside derivative
ActiveCN111072734AMild reaction systemEfficient responseSugar derivativesSugar derivatives preparationBenzoic acidFormic Acid Esters
The invention discloses a 5-deoxy-D-ribofuranose 1-[2-(1-styryl) benzoate] derivative as shown in a general formula (I) and a preparation method of the derivative, wherein the structure of the generalformula (I) is as follows, and the invention discloses a method for preparing a uracil nucleoside derivative and an antitumor drug doxifluridine by taking the derivative as shown in the general formula (I) as a raw material. The 5-deoxy-D-ribofuranose 1-[2-(1-styryl) benzoate] derivative used as a reaction raw material can be activated as a glycosyl donor under the condition of a catalytic amountof lewis acid trimethylsilyl trifluoromethanesulfonate and N-iodosuccinimide. The method avoids traditional use of equivalent or excessive lewis acid, has a mild reaction system, has no other side reaction, has efficient reaction, and has a yield up to 98%.
Owner:KUNMING INST OF BOTANY - CHINESE ACAD OF SCI
Novel method for synthesizing uridine
ActiveCN101717420ASimple post-processingReduce manufacturing costSugar derivativesSugar derivatives preparationComing outOrganic solvent
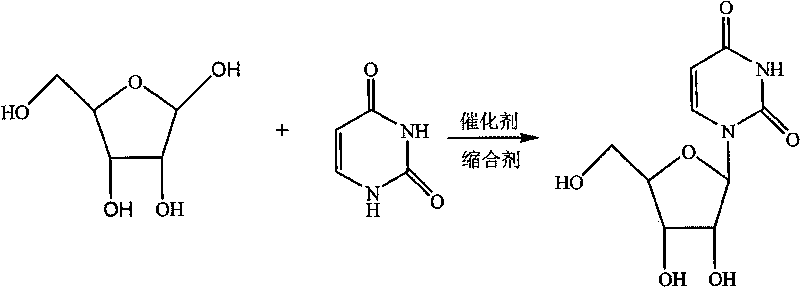
The invention discloses a novel method for synthesizing uridine. The method comprises the following steps of: adding uracil, D-ribose and a catalyst into a condensing agent, stirring and heating the mixed solution for reaction until the reaction temperature is between 20 and 80 DEG C, clarifying the mixed solution, keeping the temperature of the mixed solution for reaction for 3 to 25 hours, distilling the mixed solution at the normal pressure until the volume of the mixed solution is reduced to one fourth to one third of the original volume, after reaction, distilling the mixed solution under a reduced pressure until no liquid comes out, adding an organic solvent of which the mass is 3 to 8 times that of the D-ribose into the obtained product, stirring the mixed solution for dissolution, refluxing the mixed solution for 1 to 2 hours while stirring, cooling the mixed solution to the temperature of between 0 and 5 DEG C, crystallizing the mixed solution for 1 to 6 hours, filtering the mixed solution, and drying the filter cakes to obtain the uridine. The yield of the uridine is over 78 percent, and the HPLC of the uridine is over 99.35 percent. The method has the advantages of simple and reasonable process, good stability, safe production process, convenient operation and the suitability for industrialization.
Owner:ZHEJIANG XIANFENG TECH
Method for quickly separating and purifying monomeric compound from lindley eupatorium
InactiveCN103965276AGood reproducibilitySimple and fast operationSugar derivativesSugar derivatives preparationMedicinal herbsChemical compound
The invention discloses a method for quickly separating and purifying a monomeric compound from lindley eupatorium. The method comprises the following steps: taking dried and crushed lindley eupatorium drugs, adding the dried and crushed lindley eupatorium drugs to an ethanol solution to carry out heating reflux or normal-temperature digestion; removing ingredients such as a pigment, decocting dregs by using water, and concentrating a decoction solution in vacuum, so as to obtain a lindley eupatorium extract; separating the extract by using a high-speed counter current chromatograph, collecting a corresponding peak component according to a chromatogram map, and concentrating and drying in vacuum, so as to obtain high-purity uridine, thymine, adenine and uracil. The method is high in efficiency, simple to operate, and large in preparation quantity, and has good popularization and application value.
Owner:HUAIYIN TEACHERS COLLEGE
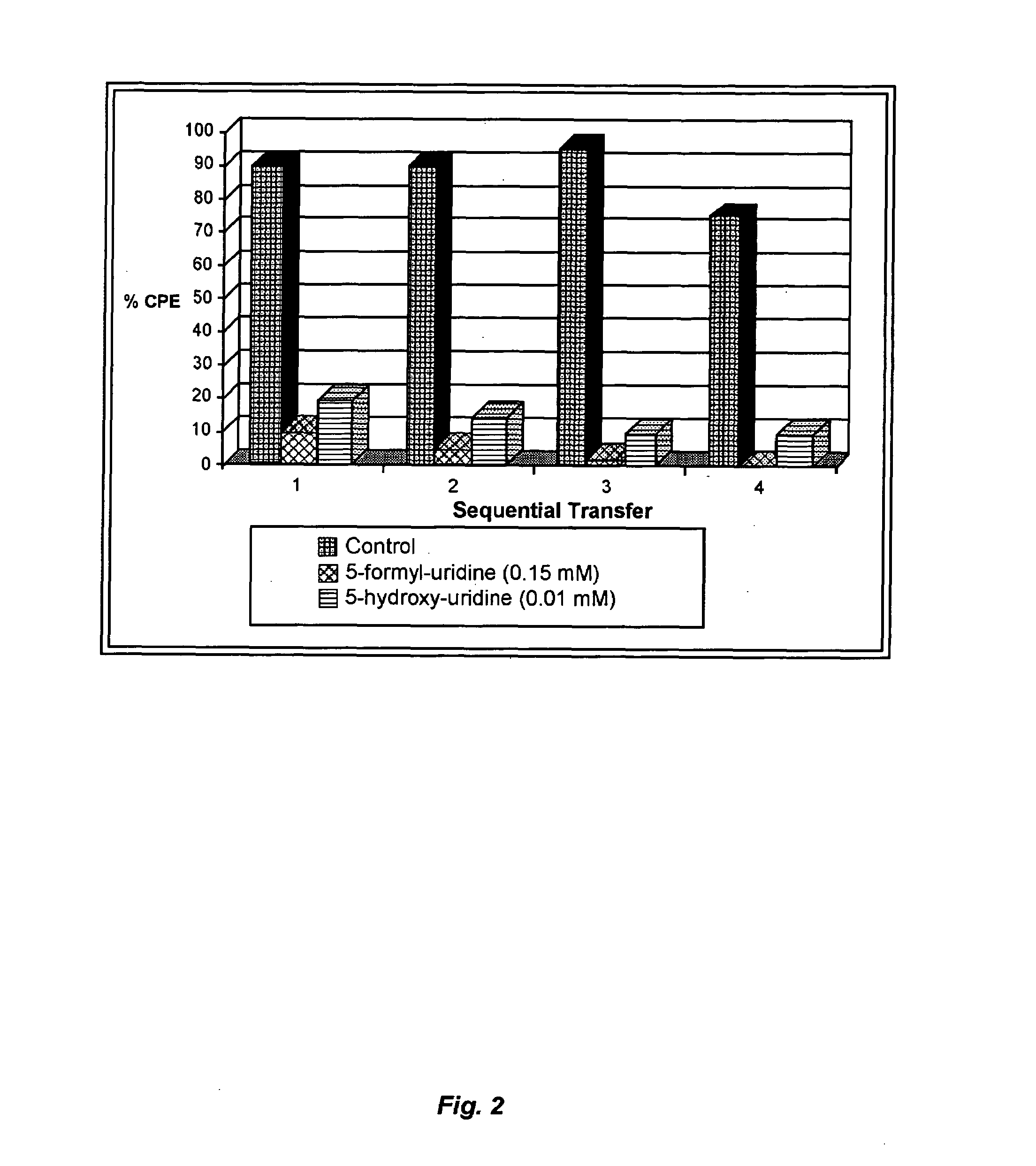
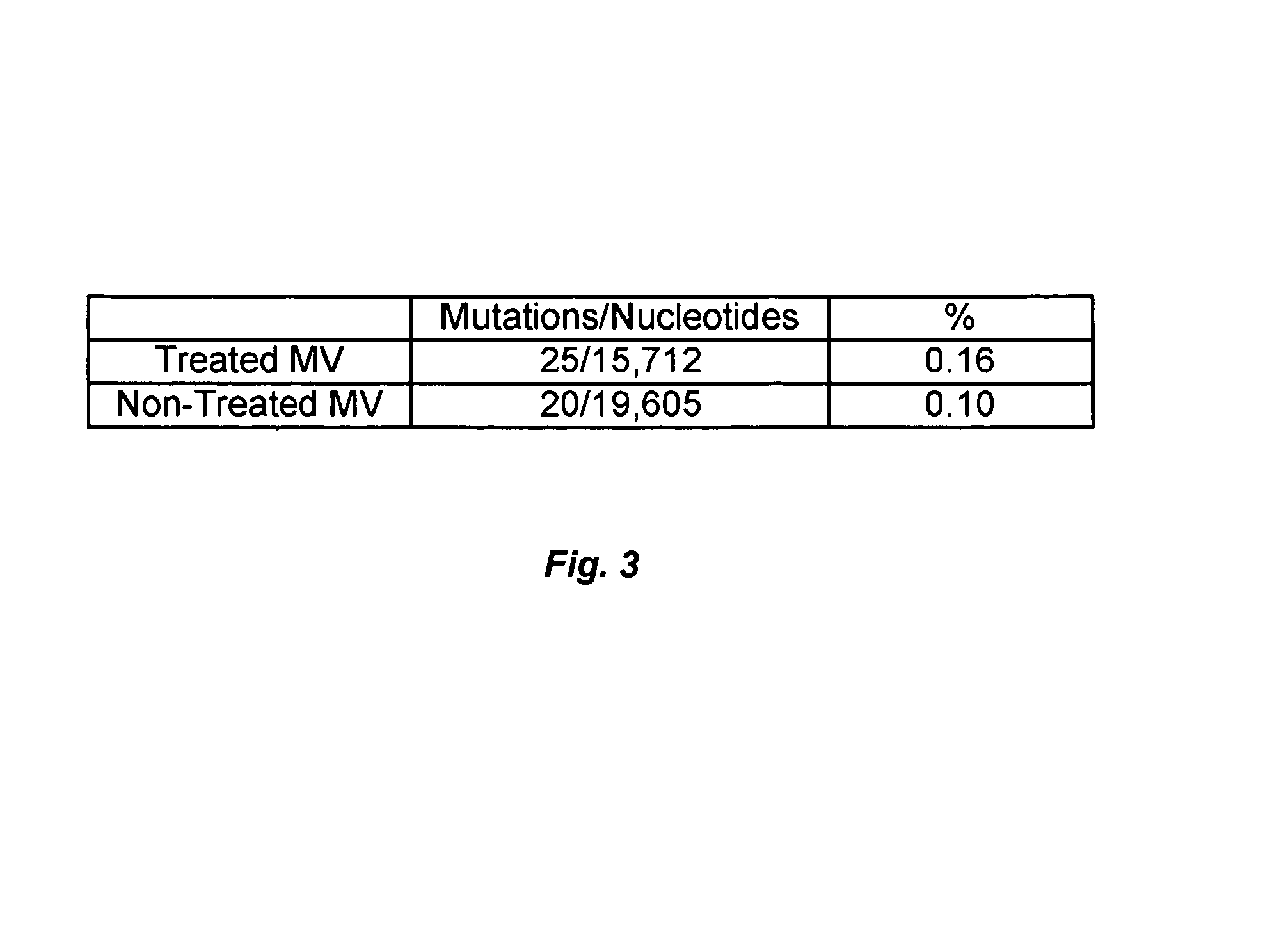
Mutagenic nucleoside analogs for the treatment of viral disease
InactiveUS7244717B2High mutation rateReduced viabilityBiocideSugar derivativesInfected cellViral disease
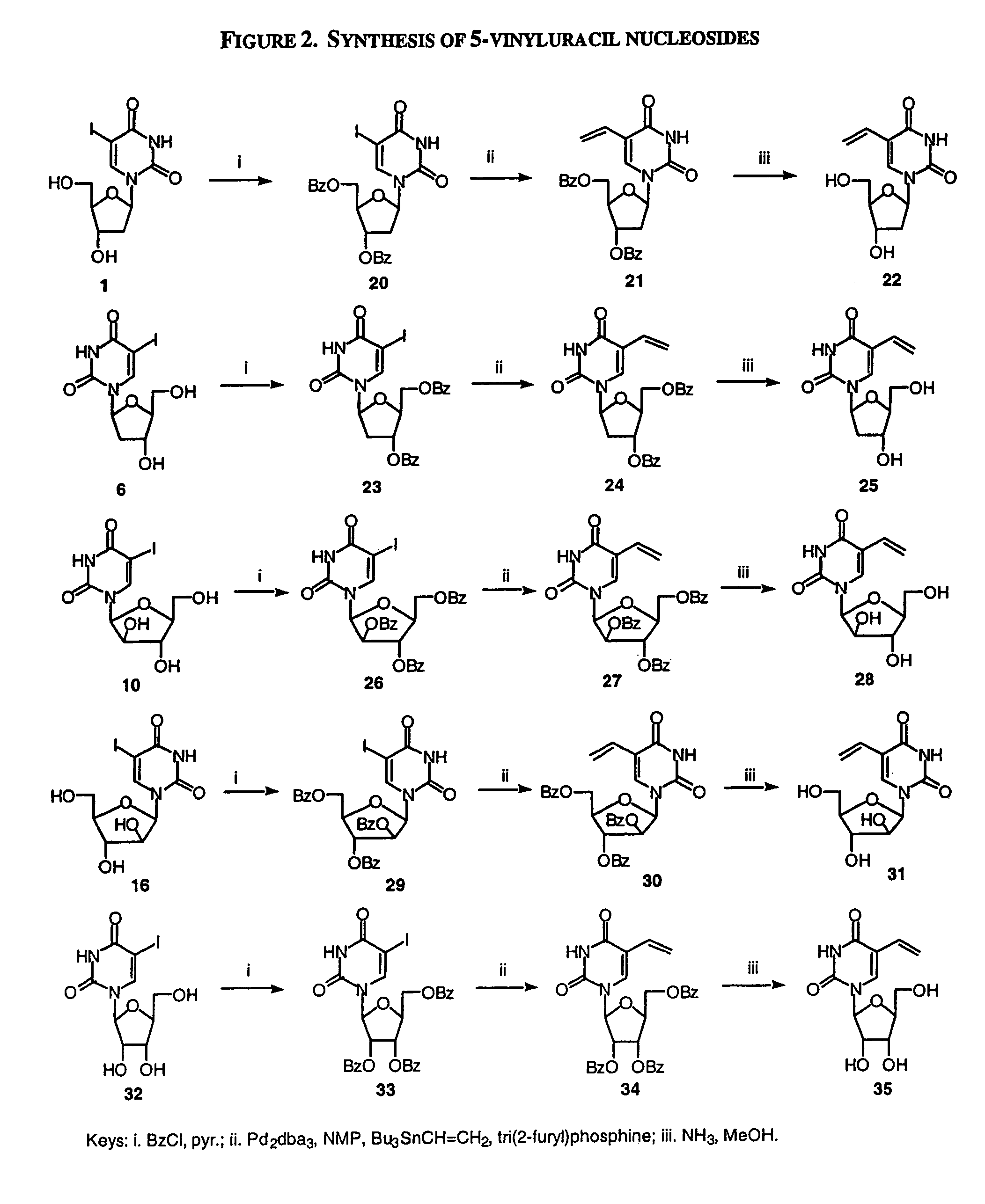
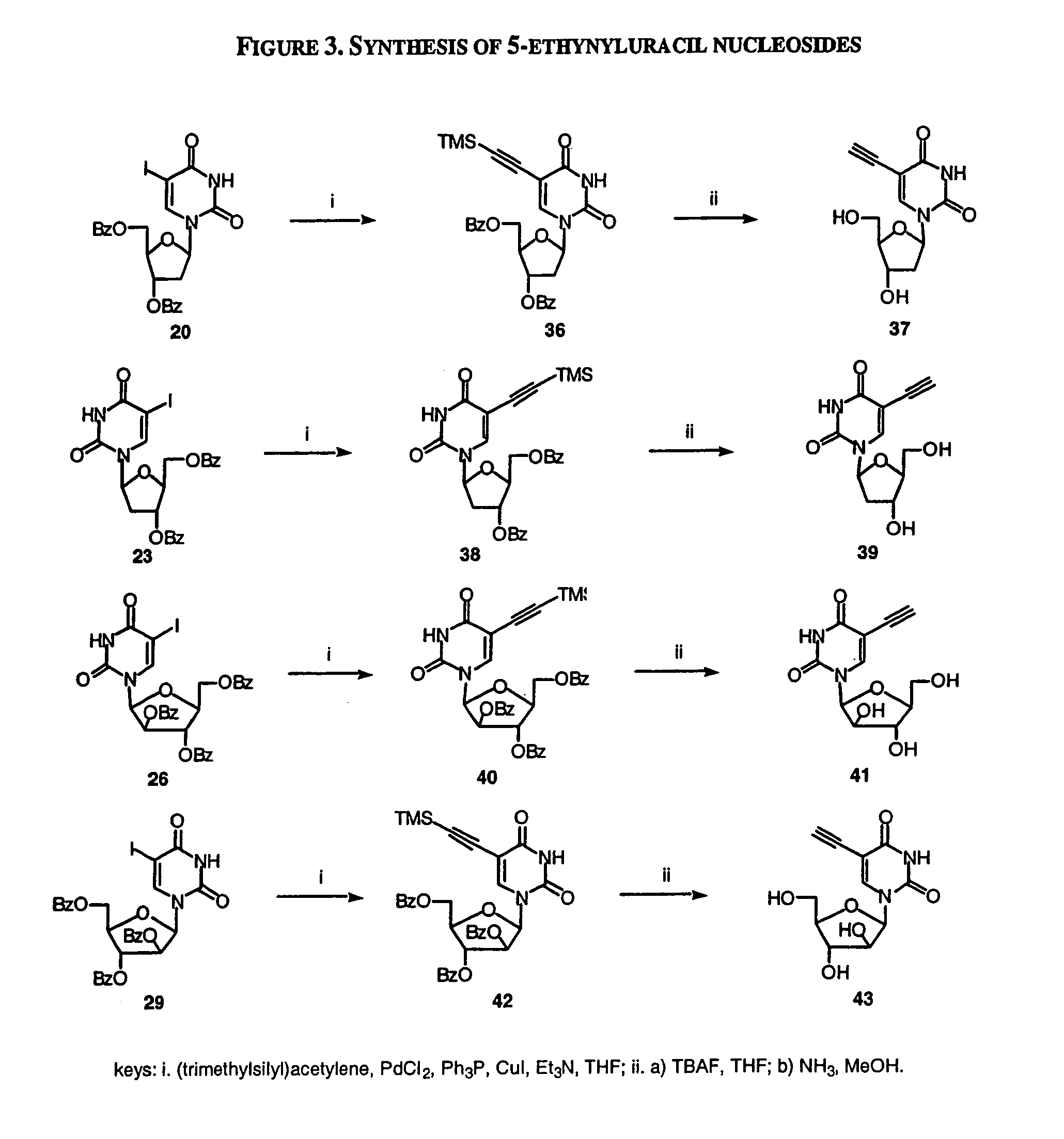
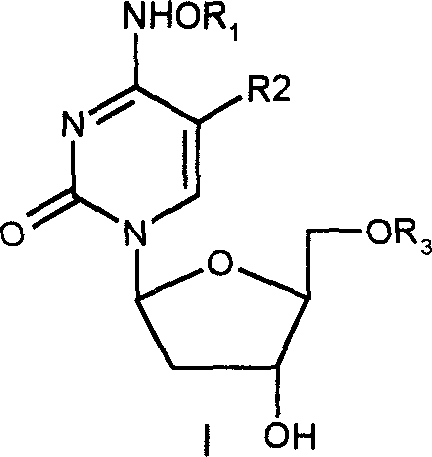
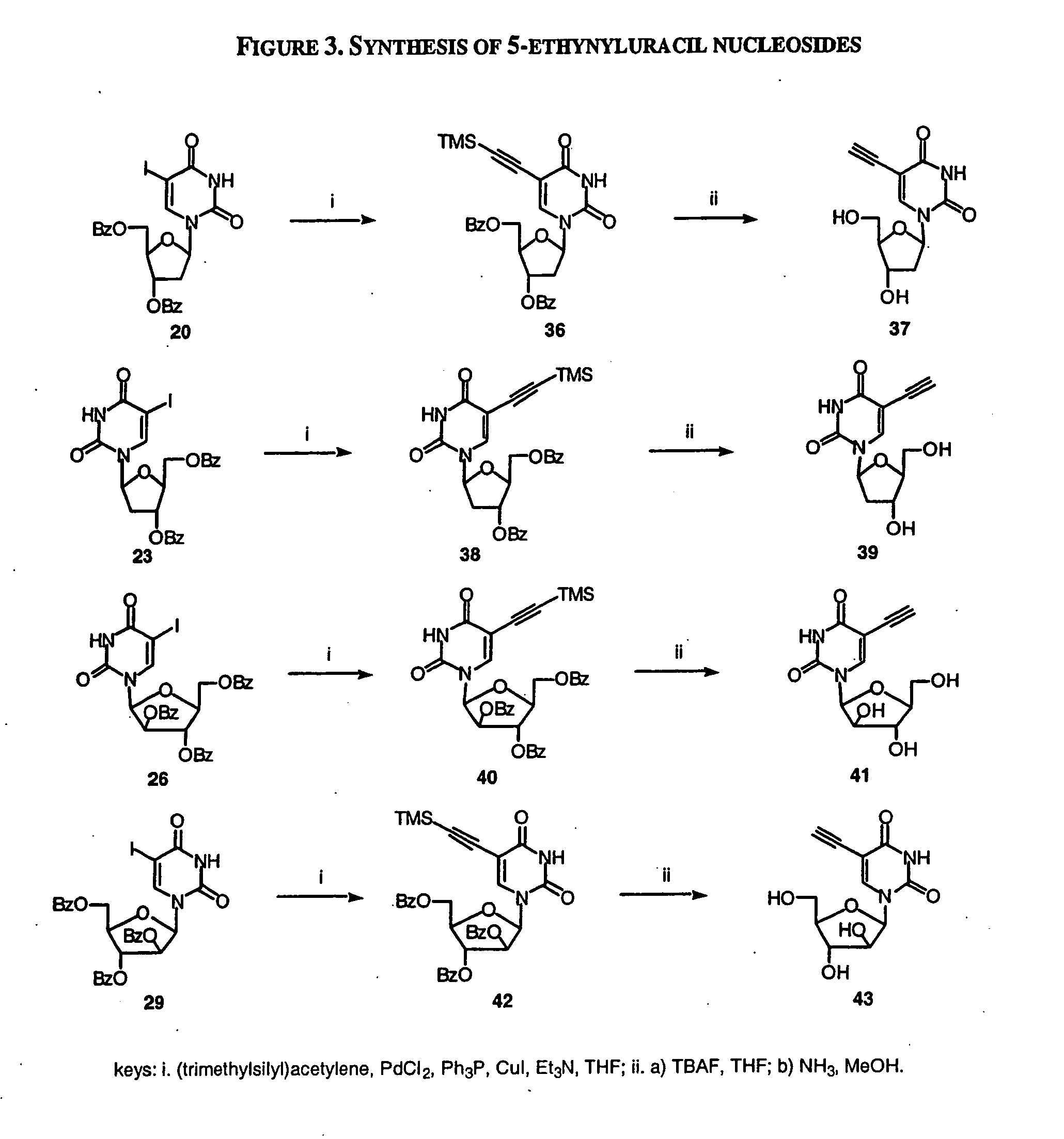
The present invention relates to methods of treating viral disease using mutagenic nucleoside analogs. In particular, the invention provides 5-aldehydo-uracil nucleosides and derivatives thereof, and methods of administration thereof to increase the virus mutation rate in a virally infected cell.
Owner:KORONIS PHARMA
Biomolecule with therapeutic tumour action and its use
InactiveCN1528776AOvercome toxic side effectsInhibition of telomerase activityOrganic active ingredientsSugar derivativesSide effectUracil nucleoside
The invention is a biomolecule curing tumours, its character: in a special sequence, it is a dichain RNA molecule with 23 basic groups. Its molecular structure contains adenine nucleotide A, guanosine G, cytidine C and uridine U. Its beneficial effects: 1, obvious effect of prohibiting tumour growth and high selectivity, able to overcome poisonous side effect of quinoline drug; 2, good stability; 3, effect amplification; 4, easy to prepare. It can be used to prepare clinical antitumor drug, where the drug form is any one of water solution injection, liposome soliquoid injection and latex.
Owner:TIANJIN SAIER BIOTECH
Compound nucleotide immunoenhancer for paralichthys olivaceus
The invention relates to a compound nucleotide immunoenhancer for paralichthys olivaceus, which is composed of the following substances in percentage by weight: 55-65% of cytidylate, 5-15% of uridine monophosphate, 5-15% of thymidine phosphorylase, 1-5% of hypoxanthine nucleotide and 10-20% of bioactive peptide. The compound nucleotide immunoenhancer disclosed by the invention is capable of obviously improving the ingestion and the growth of paralichthys olivaceus, and remarkably improving the cellular immunity, the humoral immunity, the immune gene expression quantity, the disease resistance and the like of paralichthys olivaceus. Moreover, the compound nucleotide immunoenhancer can also be directly added in a feed for paralichthys olivaceus, and orally fed; and the compound nucleotide immunoenhancer has the advantages of being free from toxic and side residues, free from drug resistance, and pollution-free to environment.
Owner:QINGDAO AGRI UNIV
Novel method for synthesizing uridine
ActiveCN101717420BThe synthesis process is simpleImprove stabilitySugar derivativesSugar derivatives preparationComing outOrganic solvent
The invention discloses a method for synthesizing uridine. The method comprises the following steps of: adding uracil, D-ribose and a catalyst into a condensing agent, stirring and heating the mixed solution for reaction until the reaction temperature is between 20 and 80 DEG C, clarifying the mixed solution, keeping the temperature of the mixed solution for reaction for 3 to 25 hours, distilling the mixed solution at the normal pressure until the volume of the mixed solution is reduced to one fourth to one third of the original volume, after reaction, distilling the mixed solution under a reduced pressure until no liquid comes out, adding an organic solvent of which the mass is 3 to 8 times that of the D-ribose into the obtained product, stirring the mixed solution for dissolution,refluxing the mixed solution for 1 to 2 hours while stirring, cooling the mixed solution to the temperature of between 0 and 5 DEG C, crystallizing the mixed solution for 1 to 6 hours, filtering the mixed solution, and drying the filter cakes to obtain the uridine. The yield of the uridine is over 78 percent, and the HPLC of the uridine is over 99.35 percent. The method has the advantages of simple and reasonable process, good stability, safe production process, convenient operation and the suitability for industrialization.
Owner:ZHEJIANG XIANFENG TECH
Preparation method of 2'-O-substituted uridine
InactiveCN110407900AReduce the difficulty of purificationImprove conversion rateSugar derivativesSugar derivatives preparationSolventUracil nucleoside
The invention discloses a preparation method of 2'-O-substituted uridine. The method comprises the steps of: mixing 2,2'-epoxyuridine, silyl ether, a Lewis acid catalyst and a solvent and reacting toobtain a compound having a structure as shown in Formula 1.
Owner:SHANGHAI ZHAOWEI TECH DEV +1
Process method for synthesizing Alzvudine
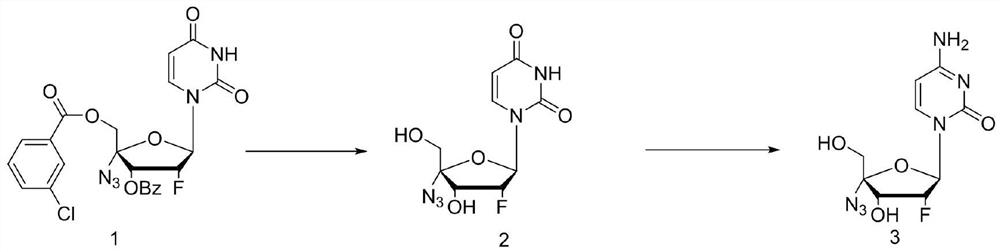
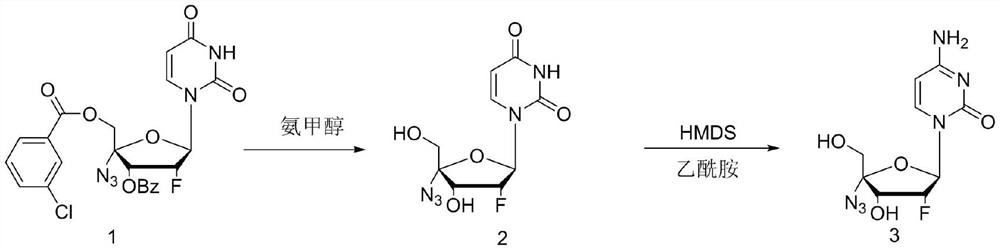
ActiveCN114149475ANovel design routeFew reaction stepsSugar derivativesSugar derivatives preparationAminolysisAlkaline hydrolysis
The invention discloses a process method for synthesizing Alzvudine, and belongs to the field of synthesis of a medical intermediate nucleoside. The method comprises the following steps: by taking a 2 '-fluoro-4'-azido-uridine intermediate as a raw material, carrying out alkaline hydrolysis to remove a protecting group, and then carrying out silyl ether aminolysis to obtain the alzovudine. The aminolysis product is obtained in one step, and reaction steps are reduced, so that the yield is improved; hexamethylsilazane and amide are adopted to directly convert 4-site hydroxyl of pyrimidine into amino, the operation is simple, less wastewater is generated in the reaction process, and environmental protection is facilitated. The whole process is simple to operate, the yield is stable in the amplification process, and the risk that the yield fluctuates and the product quality is influenced in the phosphorus oxychloride use process is avoided.
Owner:TUOXIN GROUP +2
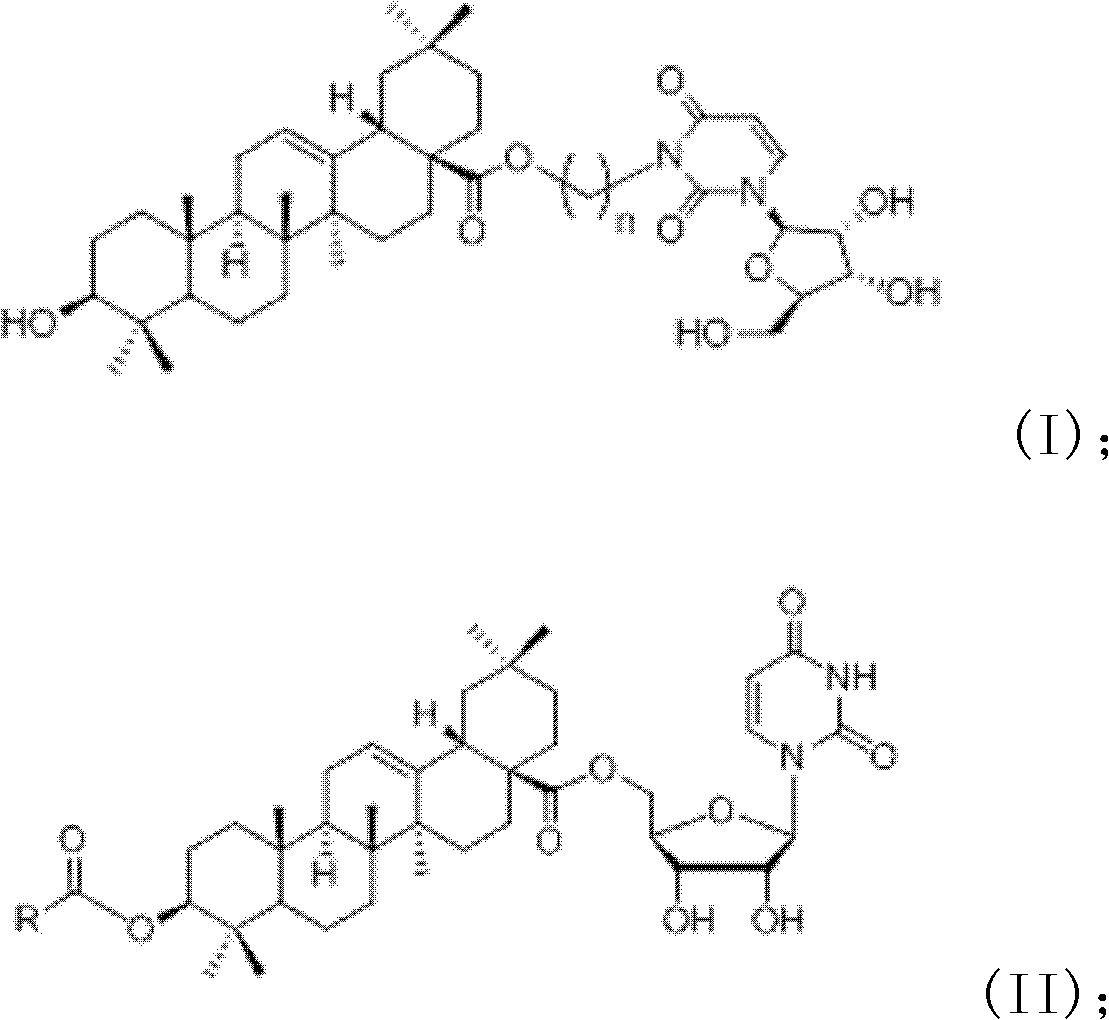
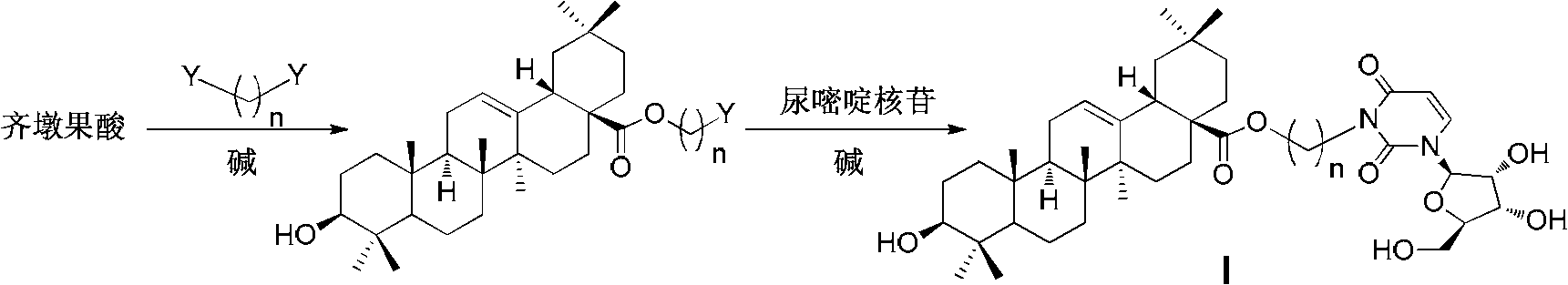
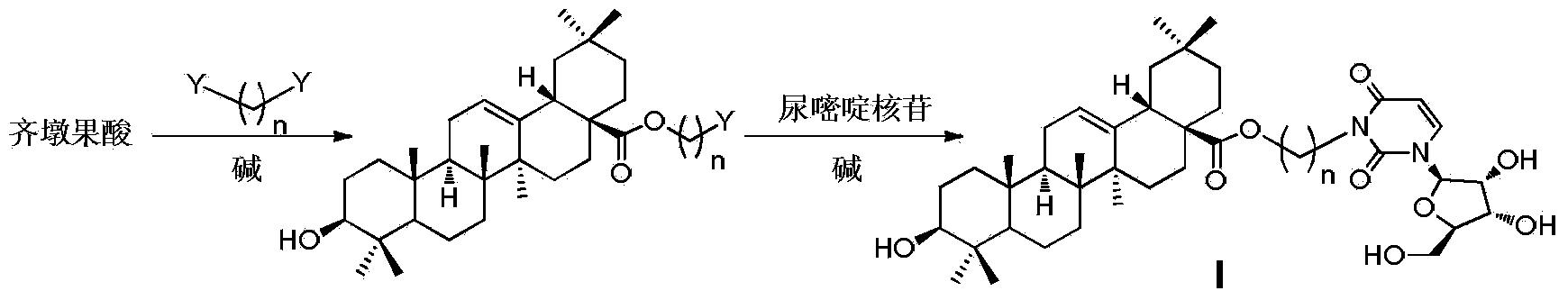
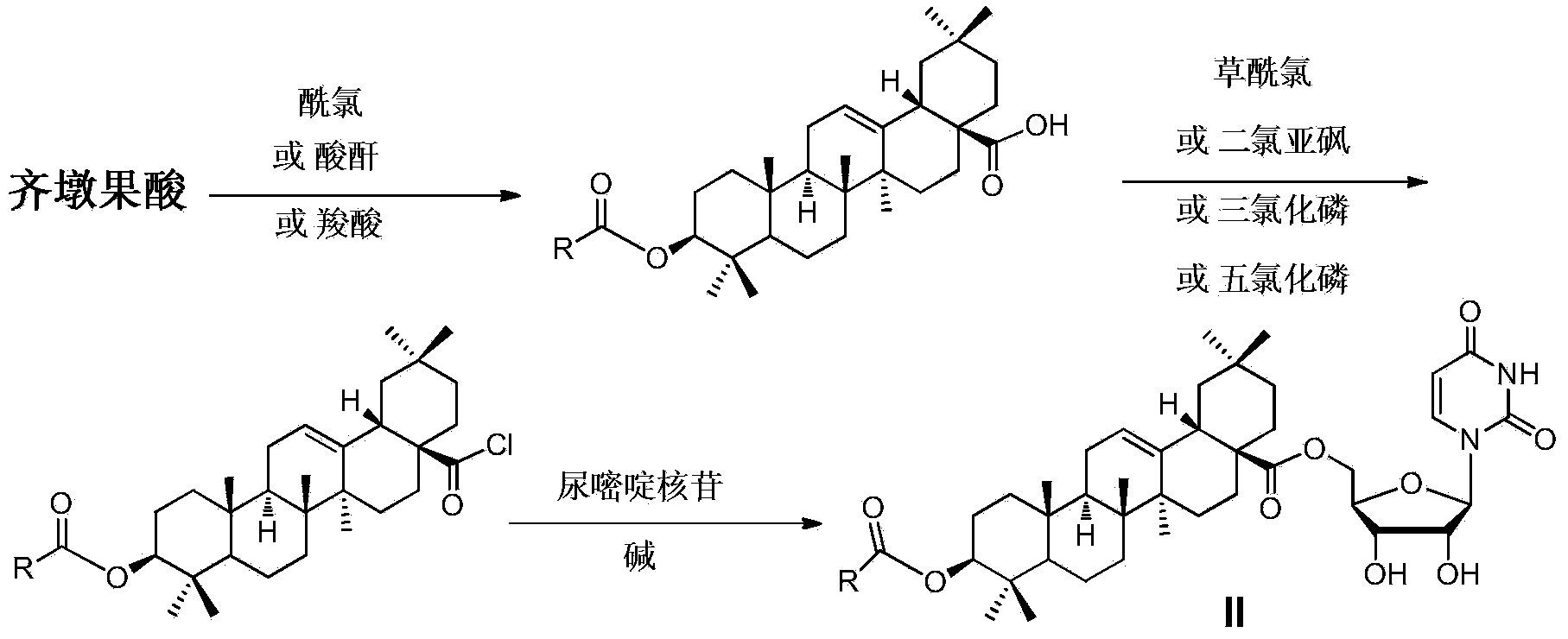
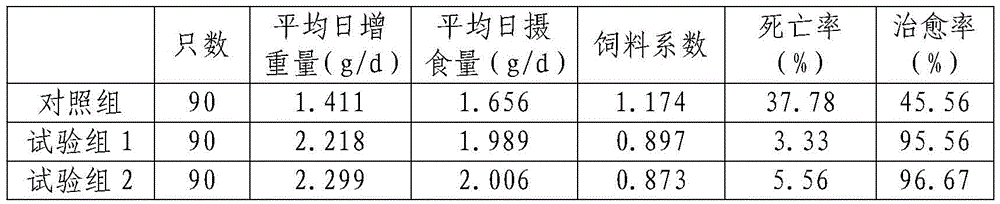
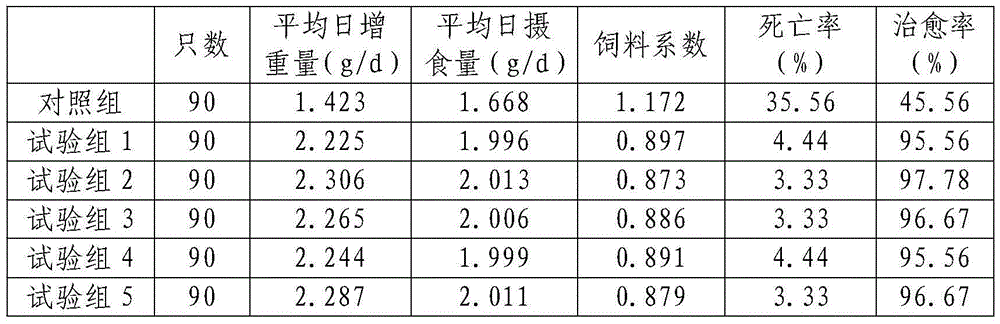
Oleanolic acid-uridine conjugate as well as preparation method and application thereof
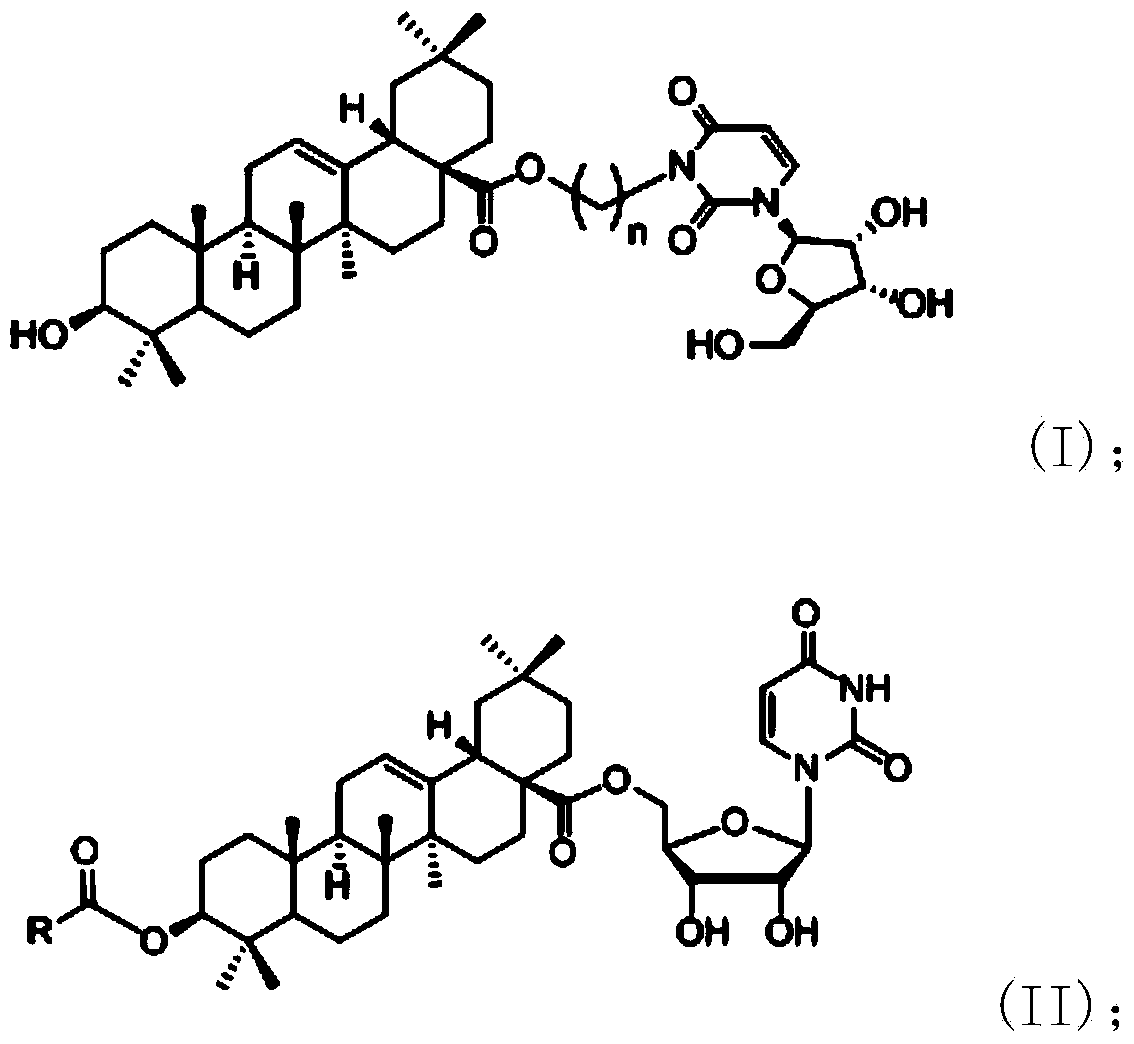
InactiveCN102633855AImprove anti-tumor activitySteroidsAntineoplastic agentsUracil nucleosideOrganic chemistry
The invention discloses an oleanolic acid-uridine conjugate and a preparation method thereof as well as application of the oleanolic acid-uridine conjugate in the pharmaceutical field. An applicant finds that the anti-tumor activity of the oleanolic acid-uridine conjugate is higher than that of a parent body namely oleanolic acid, and a lead compound is provided for developing new anti-tumor medicaments. The conjugate has a structure as shown in the following general formula (I) or (II).
Owner:GUANGXI NORMAL UNIV
Biomolecule with therapeutic tumour action and its use
InactiveCN1237070COvercome toxic side effectsInhibition of telomerase activityOrganic active ingredientsSugar derivativesSide effectUracil nucleoside
The invention is a biomolecule curing tumours, its character: in a special sequence, it is a dichain RNA molecule with 23 basic groups. Its molecular structure contains adenine nucleotide A, guanosine G, cytidine C and uridine U. Its beneficial effects: 1, obvious effect of prohibiting tumour growth and high selectivity, able to overcome poisonous side effect of quinoline drug; 2, good stability; 3, effect amplification; 4, easy to prepare. It can be used to prepare clinical antitumor drug, where the drug form is any one of water solution injection, liposome soliquoid injection and latex.
Owner:TIANJIN SAIER BIOTECH
Compound nucleotide oral liquor for improving immunity and its preparing method
InactiveCN100467028CImprove efficacyImprove immunityOrganic active ingredientsSolution deliveryUracil nucleosideAdenine nucleotide
The invention discloses a new oral liquid of compound nucleotide and preparation thereof. Each ml. of said compound nucleotide oral liquid comprising the following nucleotide in weight: adenylic acid 0.68-0.76mg, guanylic acid 0.43-0.52mg, uridylate 0.5-0.67mg, cytidylic acid 0.42-0.55mg and thymidylic acid 0.22-0.25mg.
Owner:庄田 +1
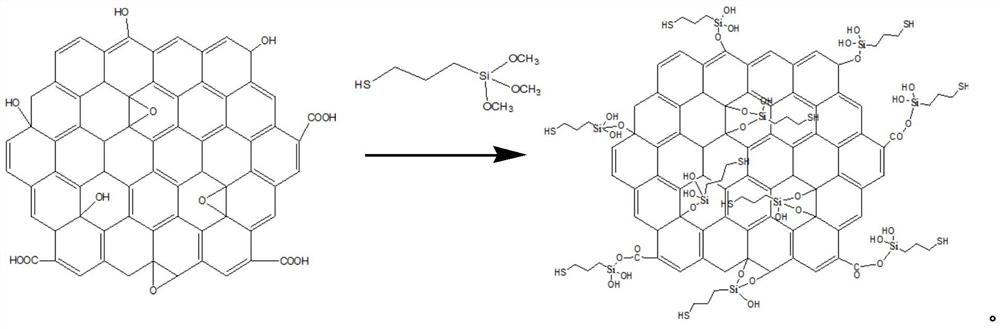
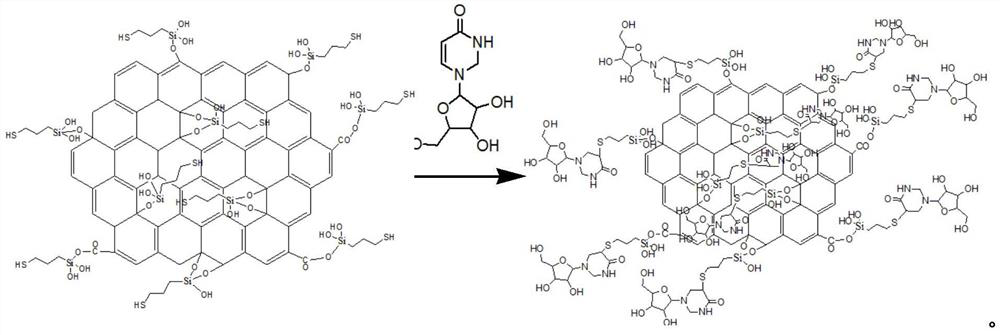
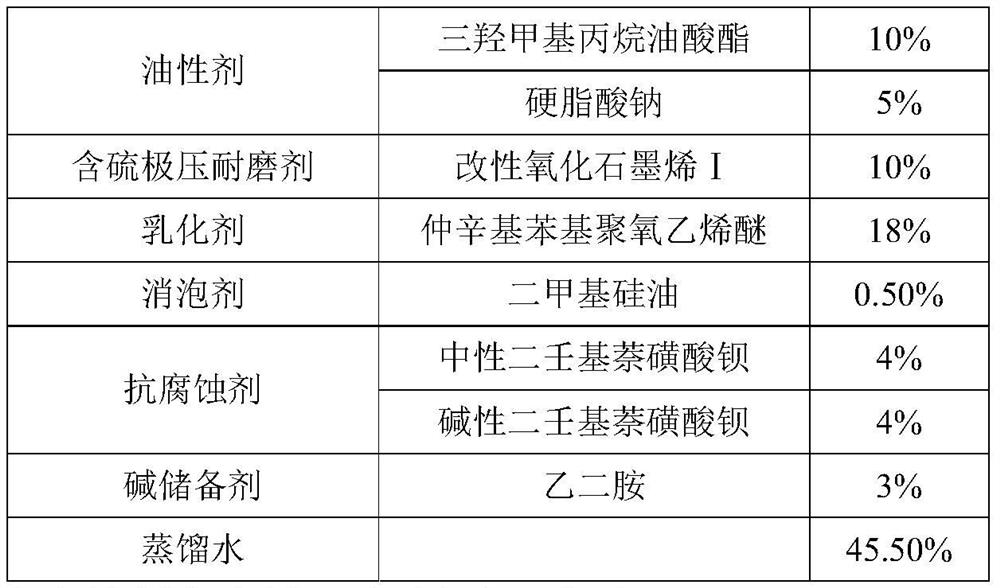
A kind of modified graphene oxide, its application, the wire drawing liquid containing it and preparation method
ActiveCN110079131BImprove extreme pressure wear resistanceImprove corrosion resistanceAdditivesPigment treatment with organosilicon compoundsUracil nucleosideMaterials science
The invention relates to the technical field of metal processing and particularly discloses a modified GO (graphene oxide), an application thereof, a drawing solution containing the modified GO and apreparation method of the modified GO and the drawing solution. The modified GO is prepared by the reaction of uracil nucleoside and an intermediate product obtained by the reaction of sulfhydryl siloxane and GO, the mass ratio of the sulfhydryl siloxane to GO is 5-10: 1, the mass ratio of the intermediate product to the uracil nucleoside is 1: 1-2, and the drawing solution contains the modified GO. The drawing solution has low sulfur content and excellent extreme pressure wear resistance, corrosion resistance and storage stability.
Owner:HEBEI UNIVERSITY OF SCIENCE AND TECHNOLOGY
Modified nucleic acid and application thereof
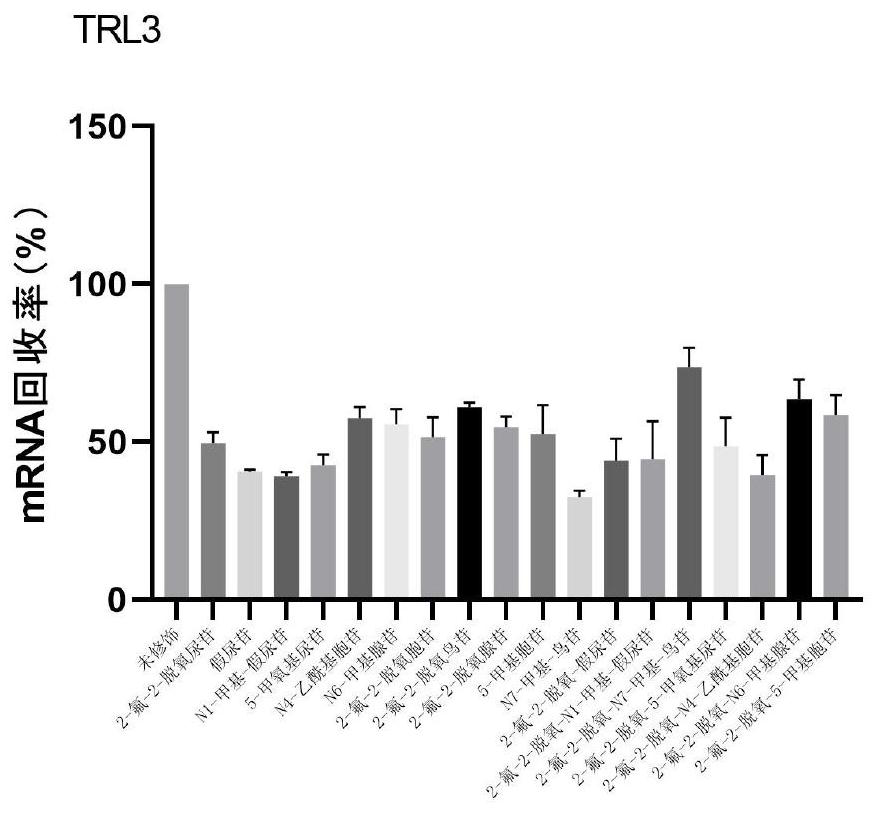
PendingCN114807154AImprove stabilityProlong half-life in vivoOrganic active ingredientsSsRNA viruses positive-senseDiseaseCytimidine
The invention provides a modified nucleic acid and application thereof, and belongs to the technical field of nucleic acid modification, the modified nucleic acid comprises uridine, cytidine, adenine nucleoside, guanine nucleoside and chemically modified nucleoside; the chemically modified nucleoside comprises one or more of chemically modified uridine nucleoside, chemically modified cytidine nucleoside, chemically modified adenine nucleoside and chemically modified guanine nucleoside. The modified nucleic acid disclosed by the invention is high in stability, low in immunogenicity and long in in-vivo half-life period; the modified nucleic acid provided by the invention can be used as a diagnostic agent or a therapeutic agent, is applied to diagnosis and treatment of diseases, overcomes the defects of low stability, high immunogenicity, short in-vivo half-life period, need of repeated administration in a short time, high cost and the like compared with the existing nucleic acid in a natural state, and reduces the application cost while enhancing the curative effect of a nucleic acid drug.
Owner:SHENZHEN RHEGEN BIOTECHNOLOGY CO LTD
Preparation method of α-uridine nucleoside
ActiveCN108424431BReduce the difficulty of purificationImprove conversion rateSugar derivativesSugar derivatives preparationSilanesCombinatorial chemistry
The invention discloses a preparation method of α-uridine nucleoside. The method comprises the steps of: after alkaline hydrolyzing the compound shown in formula C, acid treatment is carried out to obtain the compound shown in formula A; the preparation method of the compound shown in formula C comprises the steps of: making The silylated uracil is condensed with the di-esterified ribose sugar shown in formula B to obtain the compound shown in formula C.
Owner:SHANGHAI ZHAOWEI TECH DEV
Synthesis method of 5-iodouracil nucleoside
InactiveCN112010915AAvoid it happening againLow costPhysical/chemical process catalystsSugar derivativesWater methanolPtru catalyst
The invention discloses a synthesis method of 5-iodouracil nucleoside, which comprises the following steps: 1) dissolving iodine monochloride in absolute methanol, and adding a proper amount of absolute methanol; 2) adding uracil nucleoside, heating the system to 50 DEG C, and performing stirring at a rotating speed of 150 rpm for 5-8 h; and 3) stopping heating, naturally performing cooling to room temperature, performing filtering, performing washing with absolute methanol to white, and performing drying to obtain the 5-iodouracil nucleoside. According to the synthesis method disclosed by theinvention, a noble metal-containing catalyst and a controlled raw material nitric acid do not need to be used, so that the cost is greatly reduced, and the production safety is improved.
Owner:四川九元盛医药化工有限公司
Treatment of EBV and KHSV infection and associated abnormal cellular proliferation
InactiveUS20070197462A1Increases activity and bioavailability and stabilityEffective destructionBiocidePeptide/protein ingredientsMedicineDiluent
Owner:GILEAD PHARMASSET LLC
A group of six-carbocycle nucleoside analogue, its synthesis method and antiviral application
InactiveCN100412083CSaccharide with heterocyclic radicalsOrganic active ingredientsSodium bicarbonate5-fluorocytidine
The disclosed six-membered carbocyclic nucleoside analogues comprise: adenosine analogue, guanosine analogue, carnine analogue, mercaptopurine riboside analogue, cytidine analogue, 5-fluorocytidine analogue, uridine analogue, 5-fluorouridine analogue, and thymidine analogue as well as their acceptable salts formed by equimolar acid in pharmacy. Wherein, the opposite five-step synthesis method using the pinitol, acetone, methane sulfonyl chloride, p-toluenesulfonyl chloride, benzene sulfochloride, and nucleoside base as materials; the pyridine, water, glacial acetic acid, absolute methanol, DMSO, N, N-DMF as the solvent; the p-toluenesulfonic acid, 2, 2-dimethoxylpropane, anhydrous NaSO4, NaHCO3, triethylamine, and anhydrous K2CO3 as the catalyst. This invention restrains specially the replication of HIV and herpesvirus.
Owner:SHANDONG UNIV
Oleanolic acid-uridine conjugate as well as preparation method and application thereof
InactiveCN103864881AImprove anti-tumor activityOrganic active ingredientsSteroidsUracil nucleosideOrganic chemistry
Owner:GUANGXI NORMAL UNIV
Feed for treating drug-induced liver disease in tortoise and turtle as well as preparation method thereof
InactiveCN105533270APromote absorptionThe effect of absorbing this feed is goodClimate change adaptationAnimal feeding stuffAnimal scienceBetaine
The invention discloses a feed for treating drug-induced liver disease in tortoise and turtle as well as a preparation method thereof and belongs to the technical field of tortoise and turtle feed processing. The feed comprises the following raw materials in parts by weight: 40-50 parts of earthworm powder, 60-70 parts of cassava powder, 10-16 parts of table salt, 8-14 parts of dimethyl-beta-propiothetin, 6-9 parts of betaine, 5-8 parts of lysine, 5-8 parts of uridine monophosphate, 0.3-0.6 part of Tomatin polypeptide, 2-3 parts of citronellal and 24.5-42 parts of traditional Chinese medicine preparation. The preparation method of the feed comprises the following steps: drying, crushing, screening, liquid-medicine extracting, concentrating, mixing, pelletizing, sterilizing and so on. The feed integrates the functions of multiple traditional Chinese medicines, so that the feed has the effects of resisting bacteria, diminishing inflammation, reinforcing immunologic functions of the body and the like; thus, the feed is capable of effectively improving curative ratio and reducing death rate of drug-induced liver disease in tortoise and turtle. The feed disclosed by the invention is rich in nutrition as well as capable of increasing feed intake of the tortoise and turtle with drug-induced liver disease and improving the growth rate.
Owner:张莘蔓
4'-substituted carbovir-and abacavir-derivatives as well as related compounds with HIV and HCV antiviral activity
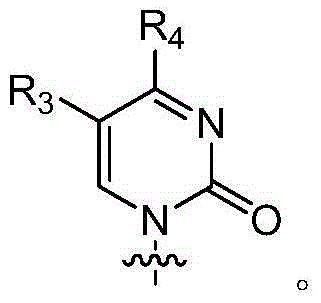
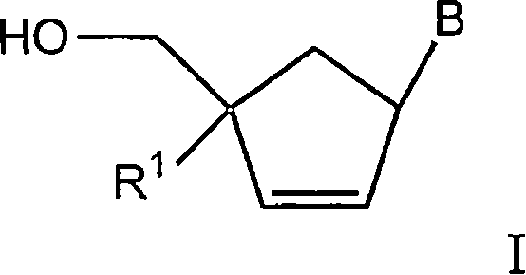
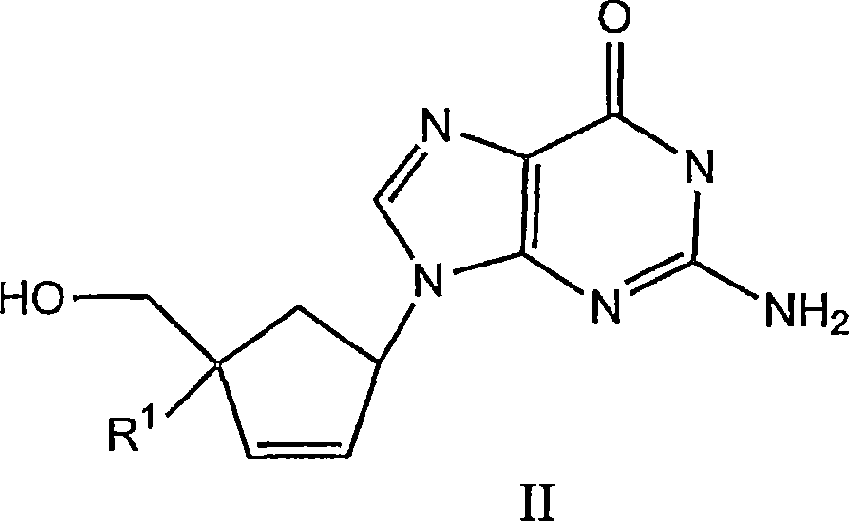
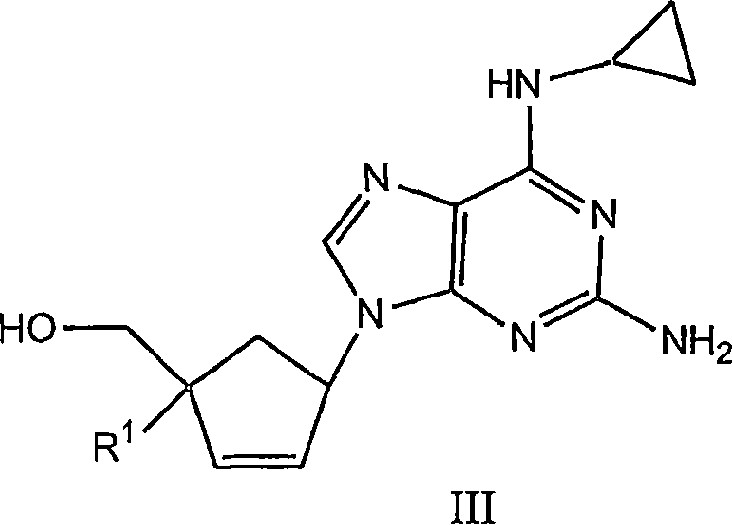
The application relates to compounds with activity against infectious viruses. Accordingly, in one embodiment the invention provides a compound of the invention which is a compound of Formula I: (I) wherein: B is adenine, guanine cytosine, uracil, thymine, 7-deazaadenine, 7-deazaguanine, 7-deaza-8-azaguanine, 7-deaza-8-azaadenine, inosine, nebularine, nitropyrrole, nitroindole, 2-aminopurine, 2-amino-6-chloropuriine, 2,6-diaminopurine, hypoxanthine, pseudouridine, pseudocytosine, pseudoisocytosine, 5-propynylcytosine, isocytosines, isoguanine, 7-deazaguanine, 2-thiopyrimidine, 6-thioguanine, 4-thiothymine, 4-thiouracil O<6> -methylguanine, N<6> -methyladenine, O<4>-methylthymine, 5,6-dihydrothymine, 5,6-dihydroucacil, 4-methylindole, triazole, or pyrazolo[3,4-d]pyrimidine; and B is optionally substituted with one or more alkyl, alkenyl, alkynyl, cycloalkyl, (cycloalkyl)alkyl, hydroxy, or halo; and R<1> is alkyl, alkenyl, alkynyl, cyano, azido, or fluoromethyl; or a pharmaceutically acceptable salt or solvate thereof.
Owner:GILEAD SCI INC
Large-scale serum-free culture method for rhIL-12 engineering cells
The invention relates to a large-scale serum-free culture method for rhIL-12 engineering cells, which comprises the following step of: inoculating rhIL-12 engineering cells in a logarithmic phase into a serum-free and protein-free medium for culture, wherein the medium is a CD CHO liquid medium comprising sodium pyruvate at the final concentration of 0.8 to 1.2mM, hypoxanthine at the final concentration of 0.075 to 0.125mM, thymidine at the final concentration of 0.012 to 0.020mM, adenosine at the final concentration of 0.5 to 0.9mg / L, guanosine at the final concentration of 0.5 to 0.9mg / L, cytidine at the final concentration of 0.5 to 0.9mg / L, uridine at the final concentration of 0.5 to 0.9mg / L, L-glutamine at the final concentration of 0.4 to 0.8mg / L, L-asparagine at the final concentration of 0.4 to 0.8mg / L, L-proline at the final concentration of 1.5 to 2.0mg / L and non-essential amino acid at the final concentration of 0.08 to 0.125mM. The invention also provides a medium used in the method. By the medium and the culture method, a high-yield and high-activity recombinant human interleukin-12 can be obtained.
Owner:UNIV OF SCI & TECH OF CHINA
Method for enzymatically synthesizing nicotinamide uracil dinucleotide
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com