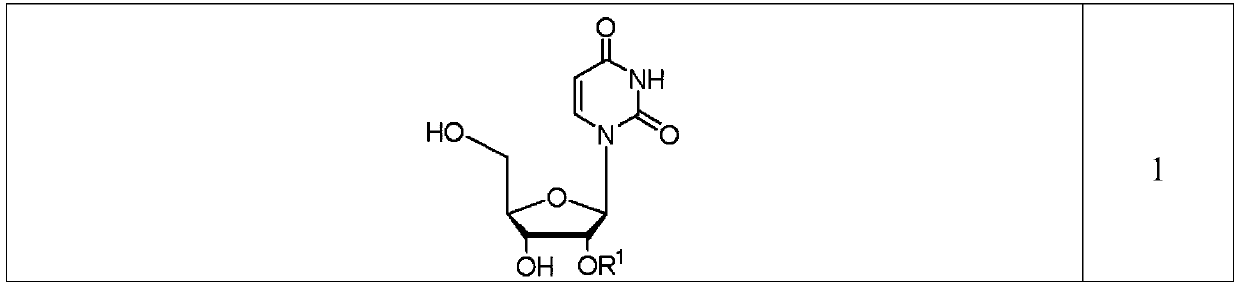
Preparation method of 2'-O-substituted uridine
A technology of epoxyuridine and compound, applied in the new synthesis field of uridine nucleoside, can solve the problems of difficult industrialization promotion, harsh reaction conditions, difficult commercialization and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0032] The reaction solvent in the preparation method of compound 1 provided by the present invention is a mixed solution, and the mixed solution is composed of the above-mentioned organic solvent 1 and organic solvent 2, and the organic solvent 2 is N,N-dimethylformamide ( DMF), dimethylacetamide (DMAc), dimethyl sulfoxide (DMSO), and N-methylpyrrolidone (NMP).
[0033] In one embodiment of the present invention, the volume ratio of organic solvent 2 and organic solvent 1 in the mixed liquid is 99:1-10:90, such as but not limited to, 90:10, 80:20, 95:5 , 70:30, 83:17, 60:40, 65:35, 50:50, 51:49, 45:55, 30:70, 25:75, 50:10, 30:10, 20:10, 45 :10, 25:10, etc.
[0034] In one embodiment of the present invention, the preparation method of 2'-O-substituted uracil (compound 1) provided comprises the steps of: combining 2,2'-epoxyuridine (CYU), silicon ether, BF 3The tetrahydrofuran solution and the 6:4 DMF / Diglyme mixture are mixed to form a reaction system, and the reaction is ca...
Embodiment 1
[0056] Myristyl alcohol tert-butyl dimethyl silyl ether (C 14 h 29 OTBDMS) synthesis
[0057]
[0058] Add 1.65kg of myristyl alcohol, 577g of imidazole, and 16.5L of DMF into the reaction kettle, stir and dissolve and add 1.28kg of tert-butyldimethylsilyl chloride dropwise. Stir the reaction at 25°C for 2 hours, UPLC analysis shows that the reaction is complete, pour the reaction solution into 50L of ice water, extract once with 16.0L of ethyl acetate, wash the organic phase with saturated brine, Na 2 SO 4 Dried and spin-dried to obtain 2.54kg of pale yellow C 14 h 29 The crude product of OTBDMS was directly used in the next reaction.
[0059] Synthesis of 2'-O-tetradecyluridine (compound 1A)
[0060]
[0061] Add 560g CYU, 2.21kg C 14 h 29 OTBDMS, 2.70L DMF and 1.80L Diglyme, add 690g BF dropwise with stirring 3 / THF solution. Raise the temperature to 140°C for 16 hours, UPLC analysis showed that the reaction was complete, cooled to room temperature, poured t...
Embodiment 2
[0064] Myristyl alcohol tert-butyl diphenylsilane (C 14 h 29 OTBDPS) synthesis
[0065]
[0066] In reactor, add 1.65kg myristyl alcohol, 577g imidazoles, 16.5L DMF, after stirring and dissolving, add dropwise 2. 40 kg tert-butyldiphenylchlorosilane (TBDPSCl). Stir and react at 25°C for 2 hours, UPLC analysis shows that the reaction is complete, pour the reaction solution into 50L water, extract once with 16.0L petroleum ether, wash the organic phase with saturated brine, Na 2 SO 4 Dried and spin-dried to obtain 3.55kg of light yellow C 14 h 29 The crude product of OTBDPS was directly used in the next reaction.
[0067] Synthesis of 2'-O-tetradecyluridine (compound 1A)
[0068]
[0069] Add 560g CYU, 3.10kg C to the reactor 14 h 29 OTBDPS, 2.70L DMAc and 1.80L Diglyme, add 690g BF dropwise with stirring 3 / THF solution. Raise the temperature to 140°C for 18 hours, UPLC analysis showed that the reaction was complete, cooled to room temperature, poured the react...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com