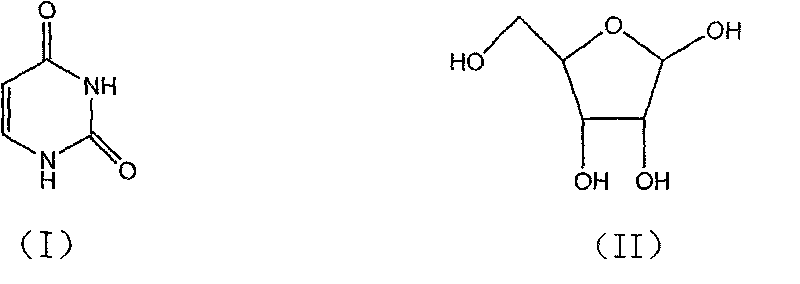
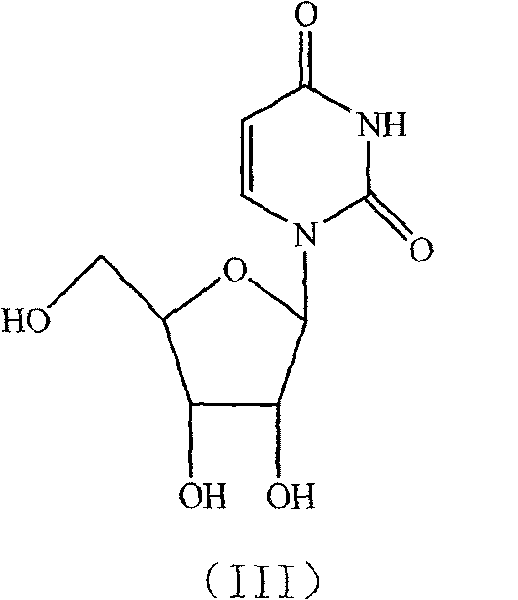
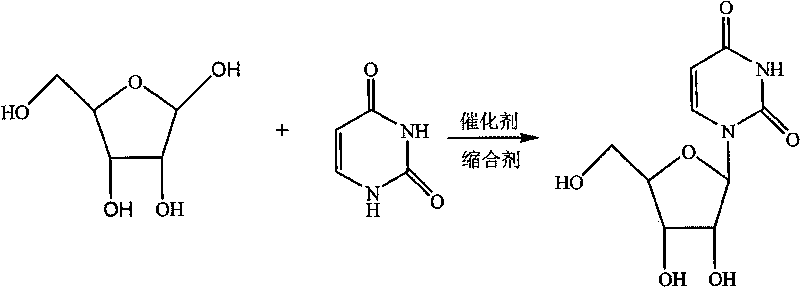
Novel method for synthesizing uridine
A technology of uridine nucleoside and a new method, which is applied in the direction of chemical instruments and methods, preparation of sugar derivatives, sugar derivatives, etc., can solve the problem of low product purity, achieve high purity, safe production process, and simple and reasonable synthesis process Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] In a dry 1000ml four-neck flask, add a reflux tube, put 74.5g of D-ribose, 56g of uracil, and 7.45g of phosphotungstic acid, dissolve in 223.5g of dichloroethane, stir and heat up, stir and clarify at 75-80°C and then keep warm React for 3 hours, distill the reaction solution to 1 / 3 of the original volume under normal pressure, then distill under reduced pressure until no liquid comes out, add 300g of ethanol, stir and heat to reflux for 2 hours, cool to below 0-5°C, stir and crystallize for 1 hour, Suction filtration and drying to obtain 95.6 g of uridine as a white crystal powder. Yield 78.9%, HPLC 99.40%.
Embodiment 2
[0020] Take 74.5g of D-ribose, 56g of uracil, and 3.5g of phosphotungstic acid, dissolve them in 230g of dichloromethane, stir at 20-22°C for clarification, keep warm for 25 hours, then distill at atmospheric pressure to 1 / 3 of the original volume, and then depressurize Distilled until no liquid came out, added 592g of methanol, stirred and heated to reflux for 2 hours, cooled to below 0-5°C, crystallized for 6 hours, filtered with suction, and dried to obtain 102.6g of uridine as a white crystal powder with a yield of 84.7%. HPLC99.57%.
Embodiment 3
[0022] Take 74.5g of D-ribose, 56g of uracil, and 3g of phosphotungstic acid, dissolve them in 447g of dichloromethane, stir and heat up to 35-40°C, stir and dissolve, then keep warm for 12 hours, and then distill at normal pressure to 1 / 4 of the original volume. Distill under reduced pressure until no liquid comes out, add 300g of ethanol, stir and heat under reflux for 1h, cool to below 0-5°C, crystallize for 3 hours, filter with suction, and dry to obtain 110.7g of uridine as a white crystal powder. The yield is 91.34%, HPLC 99.54%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com