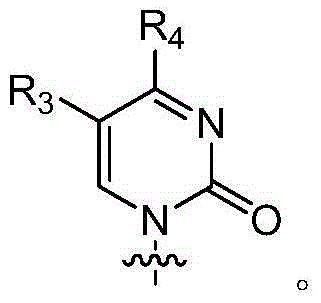
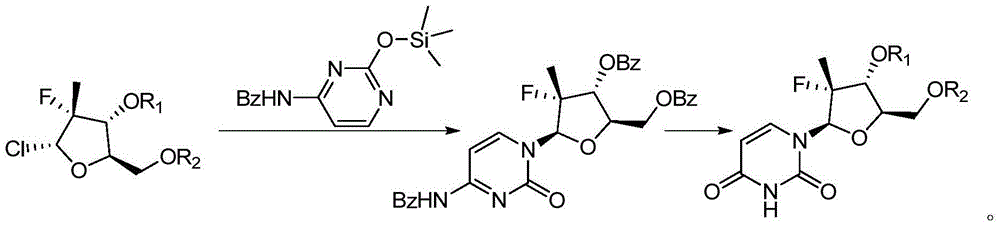
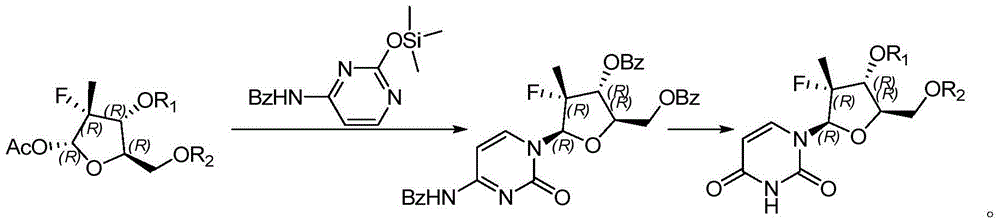
Preparation method for uracil fluoride nucleoside analogue
A technology for synthesizing uridine and analogs, which is applied in the field of preparing fluorinated uridine analogs, can solve the problems of low reaction yield, unsuitability for industrial production, and high requirements for corrosion resistance, and achieves high reaction yield , The effect of easy control of reaction conditions and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029]
[0030] Dissolve 30g of the compound shown in formula II-1 in 100mL of tetrahydrofuran, then lower the temperature to 0-10°C, add 20.4g of the compound shown in formula III-1 and 17.1g of titanium tetrachloride, raise the temperature to reflux, and keep the reflux to react to the formula The compound shown in III-1 reacted completely (about 5 to 20 hours); add saturated aqueous sodium bicarbonate solution to adjust the pH value of the reaction system to 8 to 9, carry out liquid separation, collect the organic phase, concentrate the organic phase under reduced pressure, and use methanol After purification and other post-treatments, 15.35 g of the compound represented by formula I-1 was obtained, with a molar yield of 61.5% and an HPLC purity of 99.1%.
Embodiment 2
[0032]
[0033] Dissolve 30g of the compound shown in formula II-2 in 100mL of toluene, then lower the temperature to 0-10°C, add 26.8g of the compound shown in formula III-2 and 19g of titanium tetrachloride, raise the temperature to reflux, and keep the reflux to react to formula III The compound shown in -2 reacts completely (about 5 to 20 hours); add saturated aqueous sodium bicarbonate solution to adjust the pH value of the reaction system to 8 to 9, carry out liquid separation, collect the organic phase, concentrate the organic phase under reduced pressure, and refine with methanol After post-processing, 16.9 g of the compound represented by formula I-2 was obtained, the molar yield was 59.6%, and the HPLC purity was 98.9%.
Embodiment 3
[0035]
[0036] Dissolve 30g of the compound shown in Formula II-3 in 100mL of dichloromethane, then cool down to 0-10°C, add 36.2g of the compound shown in Formula III-3 and 32.0g of trimethylsilyl trifluoromethanesulfonate, and heat up To reflux, keep the reflux reaction until the compound shown in formula III-3 reacts completely (about 5 to 20 hours); add saturated aqueous sodium bicarbonate solution to adjust the pH value in the reaction system to be 8 to 9, carry out liquid separation, collect the organic phase, After concentrating the organic phase under reduced pressure and refining with methanol, 21.8 g of the compound represented by formula I-3 was obtained, with a molar yield of 58.3% and an HPLC purity of 98.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com