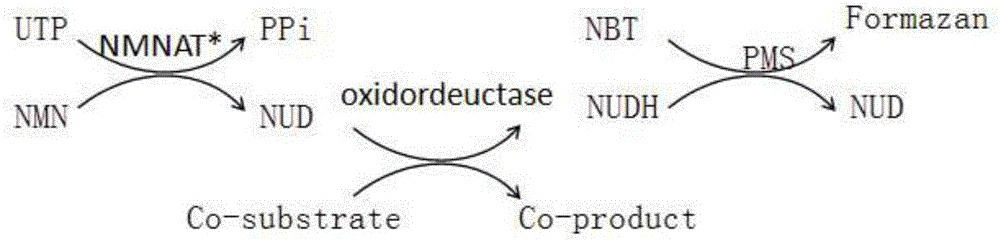
Method for enzymatic synthesis of nicotinamide uracil dinucleotide
A technology of uracil dinucleotide and uridine triphosphate, which is applied in the field of enzymatic synthesis of nicotinamide uracil dinucleotide, which can solve the problems of difficult separation and purification, difficulty of NUD entering cells, and cumbersome steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0071] Example 1 NadD 3G10 Catalytic synthesis of NUD
[0072] 1. Construction of mutant library
[0073] The mutation library was constructed by RF cloning (F. van den Ent, et al. Journal of Biochemical and Biophysical Methods. 2006, 67, 67). In the first step, the template is a wild-type NadD expression vector (see Comparative Example 1), and the primers replace the base of the amino acid site to be mutated with a degenerate base NNK, and the PCR product is recovered; in the second step, the template is the same as the first step, The primers were the PCR products recovered in the first step, and the obtained products were digested with DpnI restriction enzyme, and transferred into BL21(DE3) competent cells, and the obtained transformants were the mutant library expressing NadD mutants, each mutant The expression bacteria were named BL21(DE3)(pET-NadD-xxx), and the expressed protein product was named NadD xxx . Among them, xxx is a combination of numbers and letters, and...
Embodiment 2
[0080] Example 2 NadD 3G10 Pure Enzyme Preparation of NUD
[0081] 10mL pure enzyme NadD 3G10 The reaction system for synthesizing NUD contains Tris 50mM, UTP 2mM, NMN 4mM, MgCl 2 10mM, MnCl 25mM, pure enzyme NadD 3G10 10mg, pH 8.0, reacted at 37°C, 200rpm for 1h, and then centrifuged at 5000g, 4°C for 15min to remove protein with an ultrafiltration centrifuge tube, reference method (D.Ji, et al.Journal of the American Chemical Society.2011 , 133, 20857), isolated and purified to obtain 5.8 mg of NUD with a yield of 45%.
[0082] Product NMR data: 1 H NMR (D2O, 400MHz): δ9.36(s, 1H), 9.20(d, J=6.2Hz, 1H), 8.88(d, J=8.1Hz, 1H), 8.22(pseudo t, J=6.6Hz , 1H), 7.79(d, J=8.2Hz, 1H), 6.10(d, J=5.4Hz, 1H), 5.82-5.80(m, 2H), 4.52(brs, 1H), 4.48-4.46(m, 1H), 4.39-4.37(m, 1H), 4.34-4.32(m, 1H), 4.25-4.21(m, 2H), 4.18-4.15(m, 3H), 4.07-4.04(m, 1H).13CNMR( D2O, 100MHz): δ166.1, 165.6, 151.7, 146.1, 142.6, 141.7, 139.9, 133.9, 128.7, 102.5, 99.9, 88.5, 87.0, 82.9, 77.6, 73.7, 70.7,...
Embodiment 3
[0083] Example 3 NadD 3G10 Preparation of NUD from crude enzyme solution
[0084] NadD 3G10 The expression engineered bacteria BL21(DE3)(pET-NadD-3G10) was streak cultured, and a single colony was picked and inoculated in 5mL LB (containing kanamycin 50ng / μL) liquid medium, activated overnight at 37°C and 200rpm, All of them were inoculated in 50 mL of fresh LB (containing 50 ng / μL of kanamycin and 1 mM of IPTG), and cultured at 30° C. and 200 rpm for 24 h. The cells were collected by centrifugation, added with 5 mL of cell lysate (50 mM HPEPS pH 7.5, 1% Triton X-100, 1 mg / mL lysozyme), and lysed at 37° C. for 1 h at 200 rpm. Centrifuge (12000g) for 10min, collect the supernatant to get NadD 3G10 crude enzyme solution.
[0085] 300μL crude enzyme solution reaction system contains: Tris 50mM, UTP 100μM, NMN 4mM, MgCl 2 10mM, MnCl 2 5mM, NadD 3G10 50 μL of crude enzyme solution, pH 8.0, reacted at 37°C, 200 rpm for 2 hours. Add 150 μL of ice-cold 1.2M HClO 4 After mixin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com