Uracil nucleoside derivative and method for preparing doxifluridine medicine by using uracil nucleoside derivative
A technology of uridine derivatives and derivatives is applied in the preparation of sugar derivatives, sugar derivatives, sugar derivatives, etc., and can solve the problems of many by-products, incompatible functional groups in reaction conditions, and long conversion routes of functional groups, etc. To achieve the effect of efficient reaction and mild reaction system
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
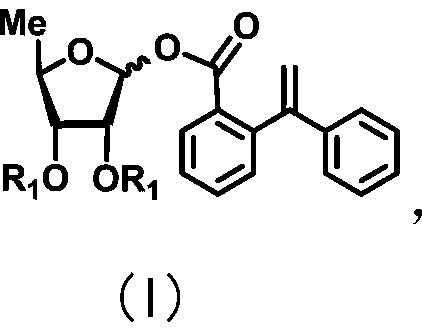
[0059] Preparation of 1-[2-(1-styryl)benzoate]-2,3-di-O-benzoyl-5-deoxy-D-ribofuranose
[0060]
[0061] Under the protection of argon, 2,3-di-0-benzoyl-5-deoxy-D-ribofuranoside (274mg, 0.77mmol), p-cresol (124mg, 1.0mmol) were dissolved in 6mL In dry dichloromethane, boron trifluoride diethyl ether (0.618 mL, 5 mmol) was then slowly added dropwise at 0°C. The reaction solution was naturally raised to room temperature and reacted for 3 hours, and the reaction was complete as monitored by thin-layer chromatography. Add triethylamine to quench the reaction, add saturated sodium bicarbonate solution, extract three times with dichloromethane, combine the organic phases, dry over anhydrous sodium sulfate, filter, and the filtrate is concentrated with a rotary evaporator, silica gel column chromatography (ethyl acetate / petroleum ether=1 / 7) to obtain 296 mg of light yellow liquid, yield 86%. The light yellow liquid obtained above was dissolved in a mixed solution of 13ml of acet...
Embodiment 2
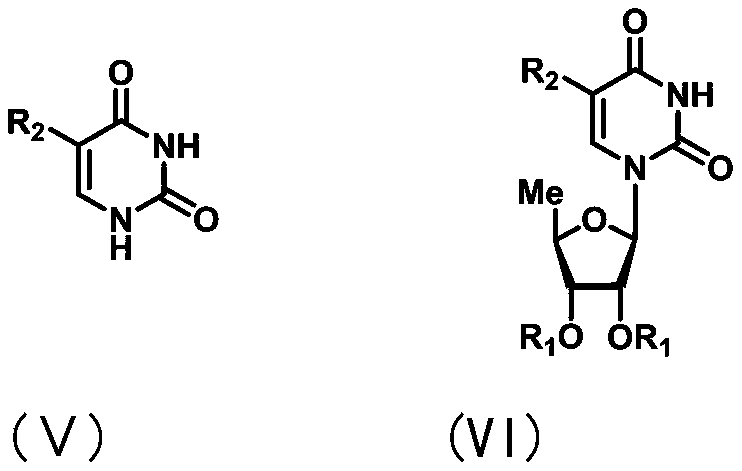
[0064] Preparation of 2',3'-di-O-benzoyl-5'-deoxy-5-fluoro-uridine
[0065]
[0066] 5-Fluorouracil (23 mg, 0.179 mmol) was suspended in 1.8 mL of anhydrous acetonitrile under the protection of argon, N, O-bis(trimethylsilyl) trifluoroacetamide (96 μl, 0.364 mmol) was added, and then The reaction solution was reacted at 50° C. for 30 minutes. Take another reaction bottle and add 1-[2-(1-styryl)benzoate]-2,3-di-O-benzoyl-5-deoxy-D-furan under the protection of argon Ribose (49mg, 0.089mmol), 360mg of 3A molecular sieves and 1.8mL of anhydrous acetonitrile, the reaction solution was stirred at room temperature for 30 minutes, then the freshly prepared uracil solution was added, and after stirring at room temperature for 15 minutes, N- Iodosuccinimide (30 mg, 0.134 mmol) and trimethylsilyl trifluoromethanesulfonate (8.0 μl, 0.045 mmol). The reaction solution was naturally raised to room temperature and continued to react for 2 hours, and the reaction was complete as monitore...
Embodiment 3
[0069] Preparation of 5'-deoxy-5-fluoro-uridine (doxifluridine)
[0070]
[0071] Under the protection of argon, 2',3'-di-O-benzoyl-5'-deoxy-5-fluoro-uridine (37 mg, 0.081 mmol) was dissolved in 0.8 mL of dry methanol, Then, sodium methoxide (4.5mg, 0.081mmol) was added at room temperature, and the reaction solution was maintained at this temperature for 1 hour, and the reaction was complete as monitored by TLC. 3N hydrochloric acid was added to adjust the pH to 5-6, and concentrated under reduced pressure to obtain the crude product, which was subjected to silica gel column chromatography (dichloromethane / methanol=10 / 1) to obtain 19 mg of white solid doxifluridine, with a yield of 95%.
[0072] [α] D 25 =4.59(c0.14,CH 3 OH); 1 H NMR (400MHz, Methanol-d 4 )δ7.73(d, J=6.5Hz, 1H), 5.76(dd, J=4.0, 1.5Hz, 1H), 4.16(dd, J=5.6, 4.1Hz, 1H), 4.00(p, J=6.3 Hz, 1H), 3.79(t, J=5.8Hz, 1H), 1.39(d, J=6.4Hz, 3H); 13 C NMR (101MHz, Methanol-d 4 )δ158.14, 157.88, 149.49, 141.65, 13...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com