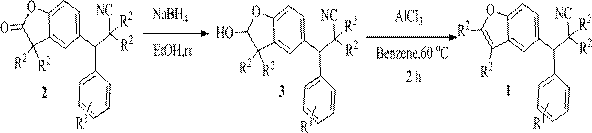
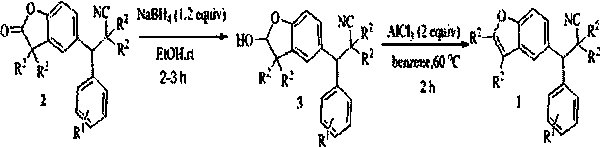
Synthetic method and application of cyanobenzofuran compound
A technology of benzofuran and synthesis method, which is applied in the direction of drug combination, organic chemistry, antineoplastic drugs, etc., can solve the problems of no literature reports, etc., and achieve the effects of good yield, easy price, and simple reaction operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0036] The present invention will be further described below in conjunction with specific examples
[0037] Raw material synthesis
[0038] The reaction raw material benzofuran-2-one compound (2a) can be prepared from 2,6-di-tert-butylphenol and benzaldehyde as starting materials, and can be obtained by the following two steps.
[0039]
[0040]
[0041] step one:
[0042] 2,6-Di-tert-butylphenol (10 mmol), benzaldehyde (10 mmol), and 40 mL of dry toluene were successively added to the Dean-Stark device, heated to reflux, and di-n-propylamine (40 mmol) was slowly added dropwise thereto. , keep the temperature unchanged, continue to reflux, and react overnight. When the reaction temperature was lowered to the boiling point of the solvent, 40 mmol of acetic anhydride was added, the solution temperature was continued to be cooled naturally, and the reaction was stirred for 15 min. The reaction solution was poured into 200 mL of ice water, extracted with DCM (3-100 mL),...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com