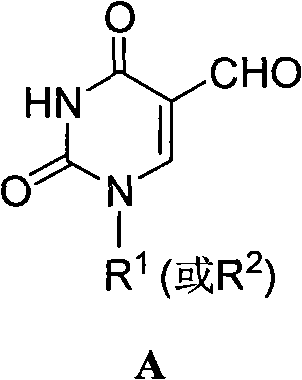
5-formacylpyrimidine carbocyclic nucleoside and preparation method thereof
A technology for formylpyrimidine carbocyclic nucleoside and formylpyrimidine carbocyclic nucleoside compound is applied in the field of 5-formylpyrimidine carbocyclic nucleoside and preparation, and achieves the effects of mild reaction conditions, simple operation process and rich reaction performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[0036] Example. Synthesis of 5-formyl-2'-deoxyuridine carbocyclic nucleoside (target product a)
[0037] (1) Add 5-methyl-2'-deoxyuridine carbocyclic nucleoside (2.40 g, 10 mmol) and DMAP (0.05 g) into a reaction flask filled with acetic anhydride (15 mL), and stir at room temperature for reaction. After the reaction is complete (monitored by TLC), add absolute ethanol, concentrate, then add a small amount of water, filter with suction, and wash with water to obtain a solid product (3.10 g), namely acetyl-protected 5-methyl-2'-deoxyuria Pyrimidine carbocyclic nucleosides. The yield was 95%.
[0038] (2) Add acetyl-protected 5-methyl-2'-deoxyuridine carbocyclic nucleoside (3.10g, 9.5mmol), potassium persulfate (5.10g) and copper sulfate (0.60g) into acetonitrile (30mL ) and water (30mL), 2,6-lutidine (3.4mL) was added, and the reaction was stirred at 65°C. After the reaction was complete (TLC monitoring), separation by column chromatography gave a white solid product (1.40 g...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com