Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
45results about How to "Moderate yield" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Process for producing arylsulfur pentafluorides
Novel processes for preparing arylsulfur pentafluorides are disclosed. Processes include reacting at least one aryl sulfur compound with a halogen and a fluoro salt to form an arylsulfur halotetrafluoride. The arylsulfur halotetrafluoride is reacted with a fluoride source to form a target arylsulfur pentafluoride.
Owner:UBE CORP
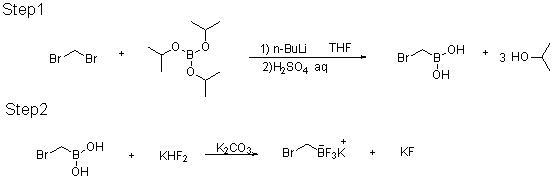
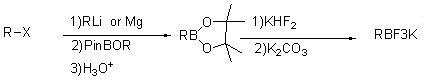
Method for preparing potassium trifluoroborate series compounds
InactiveCN102060867ASolve corrosiveAvoid affecting yieldGroup 3/13 element organic compoundsOrganic baseBoronic acid
The invention relates to synthesis of organic compounds, and provides a method for preparing potassium trifluoroborate series compounds. The method comprises the following steps of: adding organic boric acid or organic borate and solvent (THF (tetrahydrofuran), or MTBE (Methyl Tertiary Butyl Ether), or ethyl acetate, or methanol) into a reaction kettle lined with tetrafluoroethylene plastic at room temperature; adding potassium bifluoride and water at normal temperature, stirring for 1 to 12 hours, and reacting to prepare a solid-liquid mixture; adding solid potassium ion containing inorganic or organic alkali into the solid-liquid mixture after the reaction is completed, neutralizing until the pH is between 7 and 9, and continuously stirring for 1 to 5 hours; directly filtering to obtain a solid coarse product after stirring is completed; dissolving the coarse product with solvent, filtering and concentrating, adding nonpolar solvent, and pulping to obtain high-quality RBF3K series compounds. The method is easy to operate, has mild reaction conditions, and can realize scale-up production; and the product prepared by the method has high yield and excellent purity, and the cost is greatly reduced.
Owner:大连联化医药技术有限公司
Fault tolerant cell array architecture
InactiveUS20080034184A1Moderate yieldArray is largeSingle instruction multiple data multiprocessorsStatic indicating devicesMassively parallelData processing system
A data processing system containing a monolithic network of cells with sufficient redundancy provided through direct logical replacement of defective cells by spare cells to allow a large monolithic array of cells without uncorrectable defects to be organized, where the cells have a variety of useful properties. The data processing system according to the present invention overcomes the chip-size limit and off-chip connection bottlenecks of chip-based architectures, the von Neumann bottleneck of uniprocessor architectures, the memory and I / O bottlenecks of parallel processing architectures, and the input bandwidth bottleneck of high-resolution displays, and supports integration of up to an entire massively parallel data processing system into a single monolithic entity.
Owner:NORMAN RICHARD S
Synthesis method of tert-butyl-1, 7-diazaspiro [3.5] nonane-1-formate
ActiveCN111620869AReasonable reaction process designMethod route shortOrganic chemistryBulk chemical productionPalladium on carbonNonane
The invention relates to a synthesis method of tert-butyl-1, 7-diazaspiro [3.5] nonane-1-formate, and mainly solves the technical problem that no suitable industrial synthesis method exists at present. The method comprises the following seven steps: 1, reacting a compound 1 with ethyl malonate added into a solvent ethanol to obtain a compound 2; 2, reacting the compound 2 with lithium borohydridein tetrahydrofuran to obtain a compound 3; 3, reacting the compound 3 with p-toluenesulfonyl chloride in dichloromethane to obtain a compound 4; 4, adding cesium carbonate into the compound 4 in acetonitrile serving as a solvent for cyclization to obtain a compound 5; 5, adding magnesium chips into the compound 5 in methanol serving as a solvent for reduction to obtain a compound 6, 6, reacting the compound 6 with Boc anhydride in dichloromethane to obtain a compound 7, and 7, reacting the compound 7 with palladium on carbon in methanol to obtain a final compound 8.
Owner:SHANGHAI STA PHARMA R&D CO LTD +1
Preparation method of palbociclib for treating breast cancer
InactiveCN105906622AMild reaction conditionsHigh yieldOrganic chemistryAntineoplastic agentsContact reactionsDehydrogenation
The invention discloses a preparation method of palbociclib for treating breast cancer. The method comprises the following steps: 1) enabling N-cyclopentyl-2-methoxyl-3-cyan-4-methyl-5-acetyl-6-oxopiperidine-2-alkene and N-[5-(1-piperazinyl)-2-pyridyl] guanidine in the presence of N, N-Mes imidazolium salt to carry out a contact reaction to obtain 6-acetyl-8-cyclopentyl5-methyl-2-[[5-(1-piperazinyl)-2-pyridyl]amino]-5,6-dihydropyridino-[2,3-d]pyrimidine-7(8H)-ketone, wherein the temperature of the contact reaction is 85-100 DEG C; and (2) enabling 6-acetyl-8-cyclopentyl5-methyl-2-[[5-(1-piperazinyl)-2-pyridyl]amino]-5,6-dihydropyridino-[2,3-d]pyrimidine-7(8H)-ketone obtained in step 1) to carry out a dehydrogenation reaction in the presence of a dehydrogenation accelerant to generate palbociclib. The method is simple, is mild in condition and is capable of improving the yield and shortening the reaction time.
Owner:QINGDAO MUNICIPAL HOSPITAL
Synthesis method of N-(1-ethyl propyl)-3, 4-dimethylaniline
ActiveCN104250215ACo-catalyzedLow reaction temperaturePreparation by reductive alkylationDimethylaniline N-oxidePhosphoric acid
The invention discloses a synthesis method of N-(1-ethyl propyl)-3, 4-dimethylaniline, and the method comprises reaction of 3, 4-dimethyl nitrobenzene, pure hydrogen and 3-pentanone under the joint action of various catalysts at the reaction temperature of 40-55 DEG C and under the pressure reaction of 0.1MP-0.5MP hydrogen. The various catalysts comprise (a) a platinum carbon catalyst; and a (b) phosphoric acid-sodium dihydrogen phosphate acid buffer solution. The one step synthesis method of the N-(1-ethyl propyl)-3, 4-dimethylaniline is low in reaction temperature, short in reaction time and simple in process, the raw material 3, 4-dimethyl nitrobenzene can be completely transformed, and the yield of the product N-(1-ethyl propyl)-3, 4-dimethylaniline under optimized conditions is 99.8%.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Process for producing arylsulfur pentafluorides
ActiveUS20080234520A1Low costGood yieldOrganic chemistryOrganic compound preparationPentafluorideAryl
Novel processes for preparing arylsulfur pentafluorides are disclosed. Processes include reacting at least one aryl sulfur compound with a halogen and a fluoro salt to form an arylsulfur halotetrafluoride. The arylsulfur halotetrafluoride is reacted with a fluoride source to form a target arylsulfur pentafluoride.
Owner:UBE CORP
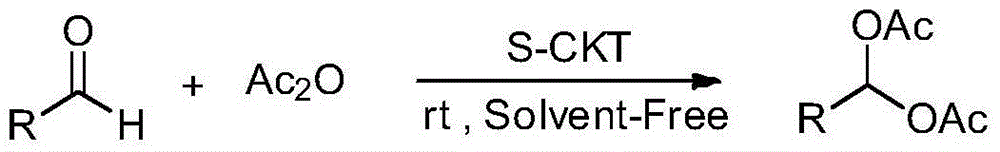
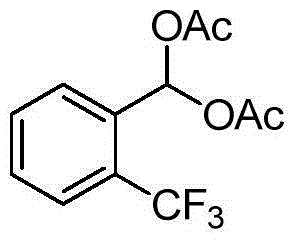
1,1-diacetate synthesis catalyzed by sulfonated cage-type mesoporous carbon
InactiveCN103319344AShort reaction timeHigh yieldOrganic compound preparationCarboxylic acid esters preparationRecyclable catalystChromatographic separation
The invention relates to 1,1-diacetate synthesis catalyzed by sulfonated cage-type mesoporous carbon. The method is characterized in that aldehyde and acetic anhydride are placed in a reactor according to a molar ratio of 1:1-3; a sulfonated cage-type mesoporous carbon catalyst is added, wherein 5-20mg of the sulfonated cage-type mesoporous carbon catalyst is added to each mole of aldehyde; the materials are stirred under room temperature until a reaction is sufficiently carried out; ethyl acetate with a volume 20-30 times that of the reaction liquid is added; the catalyst is removed by filtering; a filtrate is washed three times by using a saturated NaHCO3 water solution, and once by using water; an organic phase is dried by using anhydrous Na2SO4; the solvent is removed by reduced-pressure distillation under a pressure of 0.09MPa, such that a crude product is obtained; and re-crystallization or column chromatographic separation is carried out, such that 1,1-diacetate is obtained. Compared with existing 1,1-diacetate synthesizing method, the method provided by the invention has the advantages of short reaction time, high yield, mild reaction condition, recyclable catalyst, and the like. The method can be used for synthesizing 1,1-diacetate.
Owner:SHAANXI NORMAL UNIV
Brewing process of sweet sparkling apple wine
InactiveCN110819496AImprove easy oxidation and browningImprove qualityMicroorganism based processesAlcoholic beverage preparationReducing sugarChemistry
The invention relates to a brewing process of sweet sparkling apple wine. The process comprises the following steps: (1) soft-pressing juice taking by an extrusion method; (2) juice clarification; (3)low-temperature concentration and separation; (4) temperature-controlled fermentation; (5) pressure-maintaining low-temperature fermentation; (6) sugar-maintaining termination fermentation; and (7) clarification filtering treatment. The physicochemical indexes are as follows: the alcoholic strength is 11.5-12.0 (v / v)%, the reducing sugar content is greater than or equal to 95.0 g / L, the total acidity is 8.0-9.0 g / L, the free SO2 content is less than or equal to 50mg / L, the total SO2 content is less than or equal to 150mg / L, the volatile acid content is less than or equal to 0.8 g / L, and the pressure is 0.25-0.35 MPa. The process has the advantages that the juice is extracted through soft pressing, so that the influence of bitter substances on the aroma, taste and quality of the juice is reduced; the aroma and sugar of the fruit juice are concentrated through a low-temperature concentration technology; and through low-temperature pressure-maintaining fermentation and sugar-maintainingtermination fermentation, the finished wine is beautiful and light golden yellow. The finished wine is clear and transparent, bubbles are lasting and fine, and the finished wine has typical apple aroma and nectar aroma, strong fruity aroma, elegant and pure wine aroma, mellow and mellow taste, fullness, coordination, complexity, balance, and fragrant, fresh and lasting aftertaste.
Owner:TIANJIN AGRICULTURE COLLEGE
A kind of synthetic method of n-(1-ethylpropyl)-3,4-dimethylaniline
ActiveCN104250215BCo-catalyzedLow reaction temperaturePreparation by reductive alkylationDimethylaniline N-oxideSynthesis methods
The invention discloses a synthesis method of N-(1-ethyl propyl)-3, 4-dimethylaniline, and the method comprises reaction of 3, 4-dimethyl nitrobenzene, pure hydrogen and 3-pentanone under the joint action of various catalysts at the reaction temperature of 40-55 DEG C and under the pressure reaction of 0.1MP-0.5MP hydrogen. The various catalysts comprise (a) a platinum carbon catalyst; and a (b) phosphoric acid-sodium dihydrogen phosphate acid buffer solution. The one step synthesis method of the N-(1-ethyl propyl)-3, 4-dimethylaniline is low in reaction temperature, short in reaction time and simple in process, the raw material 3, 4-dimethyl nitrobenzene can be completely transformed, and the yield of the product N-(1-ethyl propyl)-3, 4-dimethylaniline under optimized conditions is 99.8%.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
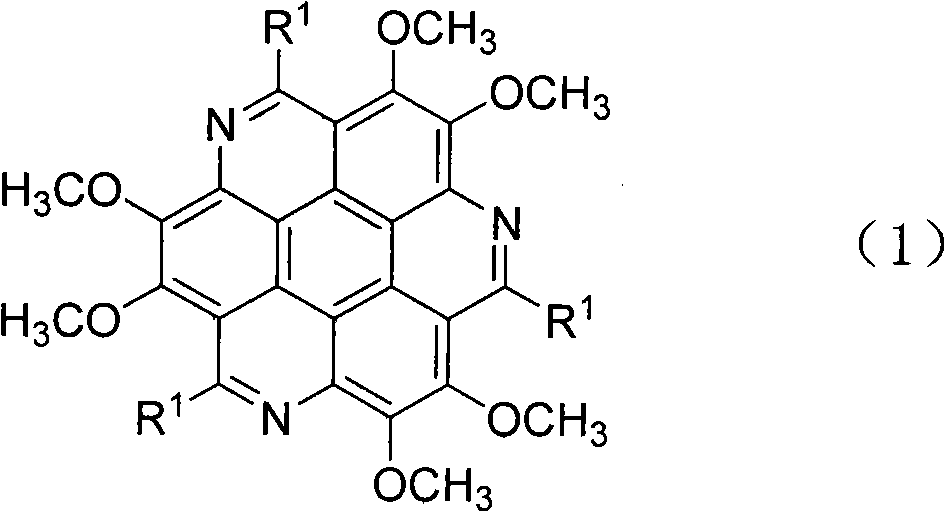
S-triazacoronene compound and synthesis method and application thereof
InactiveCN101967147BStrong fluorescenceMild reaction conditionsOrganic chemistrySolid-state devicesFluorescenceSynthesis methods
The invention relates to an s-triazacoronene compound the structural formula of which is shown in the specification. In the invention, a triamine substance is prepared from low-price available dimethoxybenzene as a raw material, and the triamine substance is catalyzed or promoted under a protonic acid to obtain various substituted triazacoronene compounds. The reaction condition is mild, the operation is convenient and the yield is moderate. The compound has stronger fluorescence in an organic solvent the fluorescence emission spectrum of which is within the range of 450-510nm, has high heat stability and chemical stability and can be used for a blue luminescent material in an organic electroluminescence device.
Owner:SHAANXI NORMAL UNIV
Brewing process of mixed fragrance type sparkling sweet apple wine
InactiveCN110938512AImprove easy oxidationPromote browningMicroorganism based processesAlcoholic beverage preparationBiotechnologyBrowning
The invention relates to a brewing process of mixed fragrance type sparkling sweet apple wine. The brewing process comprises the following steps: step 1, carrying out soft-pressing to take juice; steptwo, adjusting the acidity of lemon juice; step three, carrying out clarification of fruit juice; step four, carrying out concentration and separation at a low temperature; step five, carrying out temperature-controlled fermentation; step six, carrying out clarification and filtering; step seven, carrying out pressure-maintaining secondary fermentation; step eight, carrying out incomplete fermentation termination; step nine, carrying out low-temperature ageing; and step ten, carrying out clarification and filtration treatment under the conditions of setting physical and chemical indexes as follows: 11.5 to 12.0 (v / v) percent of the alcoholic strength, reducing sugar being greater than or equal to 95.0 g / L, total acidity of 8.0 to 9.0 g / L, free SO2 being less than or equal to 50mg / L, totalSO2 being less than or equal to 150mg / L, volatile acid being less than or equal to 0.8g / L, and the pressure of0.25 to 0.35 MPa. The brewing process has the following advantages: after the process operation, the influence of easy oxidation and browning of the apples after juicing on the aroma, taste and quality of the juice is reduced; the acidity of the apple juice is adjusted by lemon juice, sothat the complexity of the fragrance and the taste of the apple juice are increased; the oxidation resistance of the apple juice is improved; with the low-temperature concentration technology, the pressure-maintaining secondary fermentation technology, the incomplete fermentation termination technology and the low-temperature ageing technology with yeast paste, the yeast fragrance is enhanced while the wine fragrance contains the fine apple fragrance and elegant lemon fragrance, so that the aroma richness is improved, the taste is more mellow and complex, and the sweetness is pure and clean without sweet greasy feeling.
Owner:TIANJIN AGRICULTURE COLLEGE
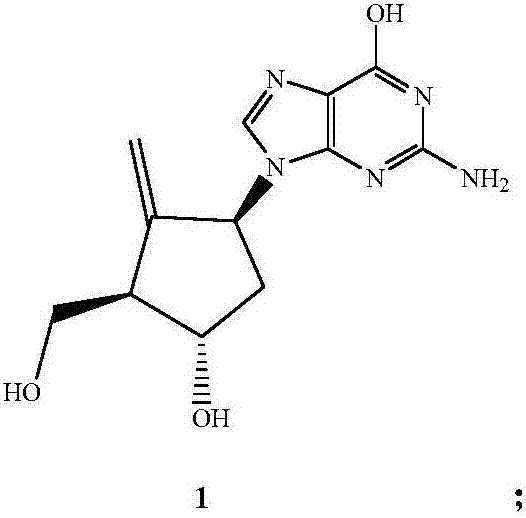
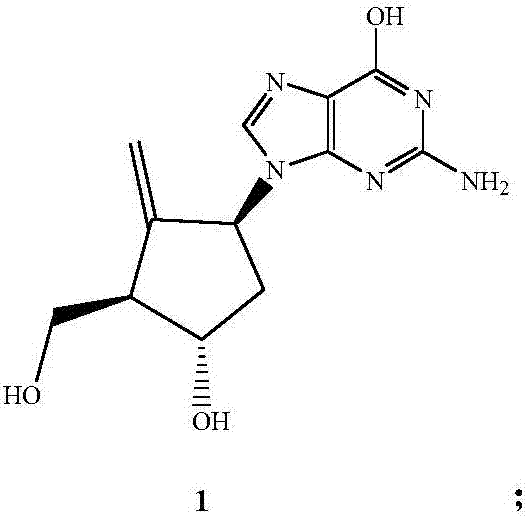
Preparation method of entecavir
ActiveCN107163049AHigh purityEasy to operateBulk chemical productionAsymmetric synthesesFuranButanedial
The invention relates to a preparation method of entecavir. The method comprises the steps: by adopting butanedial as a raw material, firstly synthesizing optically pure double-loop olefine aldehyde in the presence of a catalytic amount (R)-proline and dibenzylamine trifluoroacetate, protecting hydroxyl by using methyl, and carrying out decarbonylation by utilizing chlorotris(triphenylphosphine)-rhodium as a catalyst to obtain (3aS, 6aR)-2-methoxyl-3,3a-6,6a-tetrahydro-2H-cyclopentano [b] furan; carrying out Prins reaction in the presence of sulfuric acid to obtain a reduzate of Corey lactone, carrying out deprotection on all hydroxyls by using TBS, selectively removing a 2-bit protecting group and carrying out decarboxylic reaction in the presence of lead acetate to obtain a methylene compound; and finally introducing guanine by adopting a conventional method and carrying out deprotection to obtain the entecavir. By adopting butanedial as an initial raw material, the method has less than ten steps, and is mild in reaction condition, available in raw material and reagent, simple in operation, moderate in yield and suitable for industrial production.
Owner:HUBEI GRAND LIFE SCI & TECH CO LTD
Cell-penetrating fluorescent dyes with secondary alcohol functionalities
InactiveUS20190367737A1Improve performanceMaintaining sufficient photostabilityPyronine/xanthon/thioxanthon/selenoxanthan/telluroxanthan dyesBiological material analysisAlcoholFluorescence
The invention relates to novel cell-penetrating fluorescent dyes with secondary alcohol functionalities having one of the following general formulae I-III and 4: The invention also relates to the use of these compounds for optical microscopy and imaging techniques.
Owner:MAX PLANCK GESELLSCHAFT ZUR FOERDERUNG DER WISSENSCHAFTEN EV
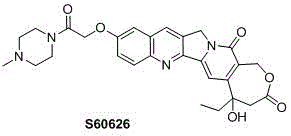
Homocamptothecin compound and synthesis method thereof
The invention relates to camptothecin, in particular relates to homocamptothecin compounds and a synthesis method thereof, and mainly solves the technical problems of poor solubility and bioavailability of homocamptothecin in the prior art. Structural formulas of the homocamptothecin compounds are as shown in I and II, and the homocamptothecin compounds comprise racemic compounds and (S)-and (R)-stereisomers corresponding to the racemic compounds. The invention specifically relates to derivation of 10-hydroxyl homocamptothecin compound, the 10-hydroxyl homocamptothecin is used as a mother nucleus for synthesis of a series of homocamptothecin ester and amide compounds. The invention provides a new synthetic route, and according to the path, a full synthesis ring enlargement method is not needed, and protection and deprotection are not needed.
Owner:SUNDIA MEDITECH COMPANY LTD
A kind of synthetic method of polysubstituted benzo [c, d] indole compound
ActiveCN107868036BReduce separation and purification processThe operation method of the reaction is simpleOrganic chemistryLuminescent compositionsSynthesis methodsDistillation
The invention discloses a synthesis method of a polysubstituted benzo[c, d] indole compound. The synthesis method comprises the following steps: taking an 8-alkynyl naphthylamine compound, a catalystand alkali according to a molar ratio of 1:(0.1-0.5):(1-2), and putting into a reaction container; adding a solvent into the reaction container until the 8-alkynyl naphthylamine compound is totally dissolved; putting the reaction container in an oil bath at 0-100 DEG C, and stirring for reacting for 0-24h; cooling to room temperature; filtering with kieselguhr or silica gel powder; flushing the filter residue with dichloromethane for 2-4 times; combining the filtrates and performing reduced-pressure distillation to remove the solvent; separating with a silica gel chromatographic column, and performing reduced-pressure distillation to obtain the polysubstituted benzo[c, d] indole compound. According to the synthesis method disclosed by the invention, the polysubstituted benzo[c, d] indole compound is synthesized by a one-pot one-step process, the processes of intermediate separation and purification are reduced, and the operation method is simple; moreover, the reaction conditions are mild, the reaction selectivity is high, the yield is high, the substrate is widely applicable, the reaction raw materials are simple and easily available, and the production cost is low; the synthesismethod is suitable for small-scale preparation in a laboratory as well as industrial large-scale production.
Owner:JIAXING UNIV
2-(4-hydroxy benzene)-methyl acetate and its preparation method
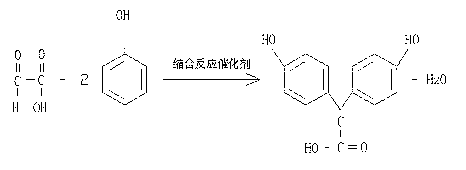
InactiveCN102249919BNo emissionsGood antibacterial effectOrganic compound preparationCarboxylic acid esters preparationBenzoic acidContinuous use
The invention relates to 2-(4-hydroxy benzene)-methyl acetate, which is prepared from the raw materials of phenol and glyoxalic acid with the steps of condensation reaction, neutralization and extraction, phenol recovery, acidification and extraction, rectification and esterification. The bacteriostatic activity of 2-(4-hydroxy benzene)-methyl acetate mainly derives from molecular-state 2-(4-hydroxy benzene)-methyl acetate. As the hydroxy within a molecular is esterified, no ionization can occur any more, so 2-(4-hydroxy benzene)-methyl acetate can exert good bacteriostatic effects when the pH ranges from 2-8. With a phenolic hydroxyl structure, 2-(4-hydroxy benzene)-methyl acetate presents a stronger antibacterial property than benzoic acid and sorbic acid. On the basis of different physicochemical characteristics, 2-(4-hydroxy benzene)-methyl acetate can be applied to anticorrosion in food, medicine, organic synthesis and other industries. And the preparation method is characterizedby short process route, mild process condition, high yield, easy implementation, environmental friendliness and no pollutant discharge. In addition, the wastewater from production can be treated for continuous use.
Owner:SHOUGUANG YUYUAN CHEM
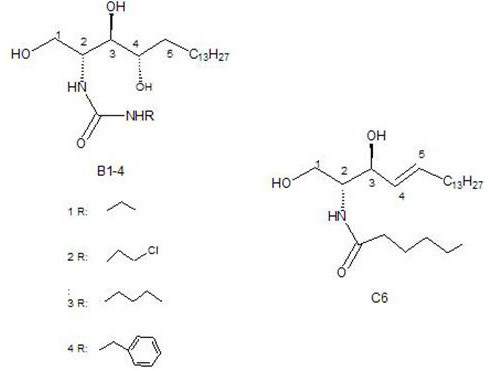
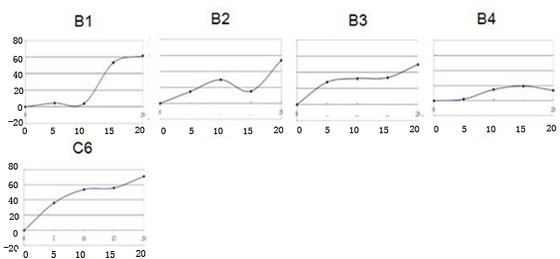
Ceramide analog b and its preparation method and application
ActiveCN109721510BSynthetic conditions are mildEasy to controlUrea derivatives preparationOrganic compound preparationPharmaceutical drugLeukemia
The invention discloses a novel ceramide analogue B, and a preparation method and application thereof, and belongs to the technical field of medicines. On one hand, the invention further provides thenovel ceramide analogue B, and on the other hand, the invention provides the preparation method and application of the novel ceramide analogue B. The synthetic condition of the novel ceramide analogueB disclosed by the invention is mild and is easy to control, the yield is moderate, and industrialization is easy. The novel ceramide analogue B provided by the method is more easily combined with enzymes, can inhibit proliferation of tumor cells, and also has obviously influence on differentiation and period of leukemia tumor cells, and multi-way tumor resistance can be facilitated.
Owner:ZHEJIANG UNIV
Truxeneone-benzophenanthrene disk-shaped liquid crystal compound and preparation method thereof
InactiveCN109575937AGood chemical stabilityImprove thermal stabilityLiquid crystal compositionsIce waterSemiconductor materials
The invention relates to a truxeneone-benzophenanthrene disk-shaped liquid crystal organic semiconductor material and a preparation method thereof. The method includes adding monobromobenzophenanthrene and potassium carbonate into N, N'-dimethylformamide, adding a trihydroxytruxene compound under the protection of inert gases, heating to the temperature of 80 DEG C to conduct a reaction for 36-48hours while stirring, removing the gas protection, and exposing the reaction system in the air to continue heating for 24-36 hours of reaction; after the reaction is completed, cooling reaction liquidto room temperature, pouring the reaction liquid into ice water to precipitate out red solids, performing suction filtration to obtain a crude product, performing vacuum drying, purifying the crude product through column chromatography isolation, taking dichloromethane as eluent, and recrystallizing a mixed organic solvent, and performing vacuum drying. The synthesis method has the advantages ofsimple operation, mild reaction condition and moderate yield, and the reactions of etherification-based polymer construction and oxidation-based truxeneone acquisition are connected in series by one step, and the p-n type disk-shaped liquid crystal compound is successively synthesized; the efficient preparation method is provided for complex truxeneone derivatives.
Owner:SICHUAN NORMAL UNIVERSITY
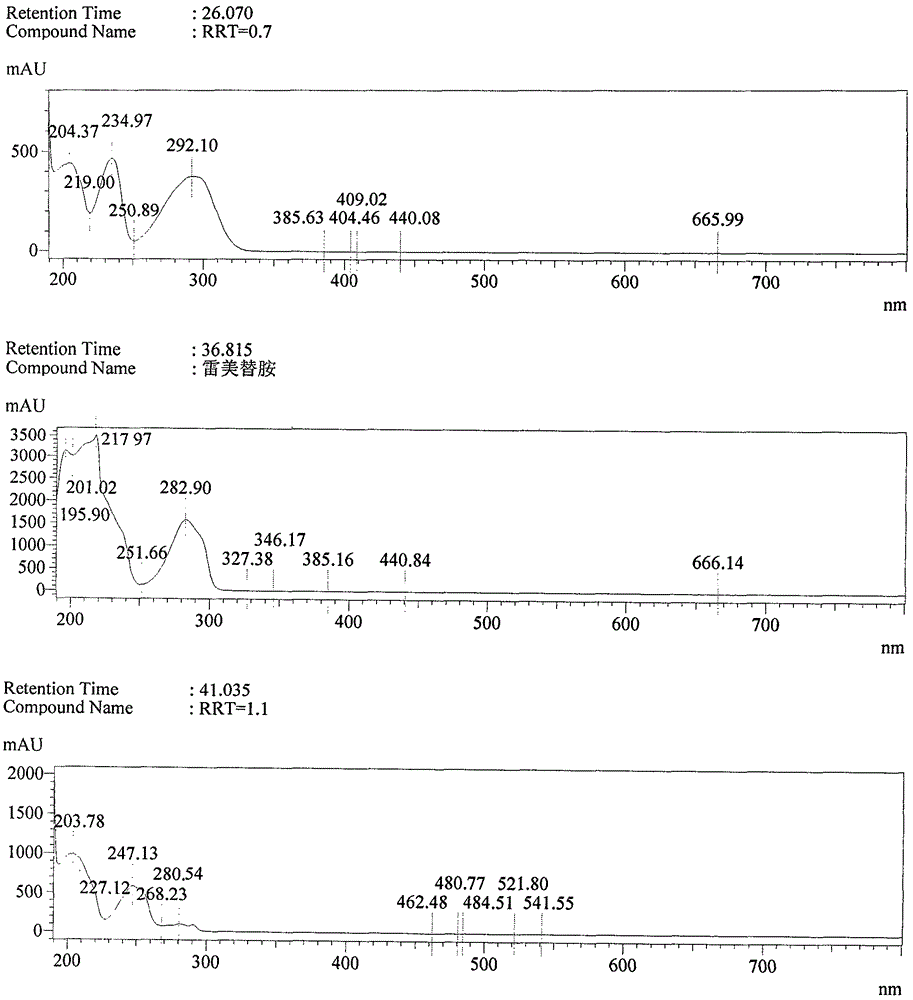
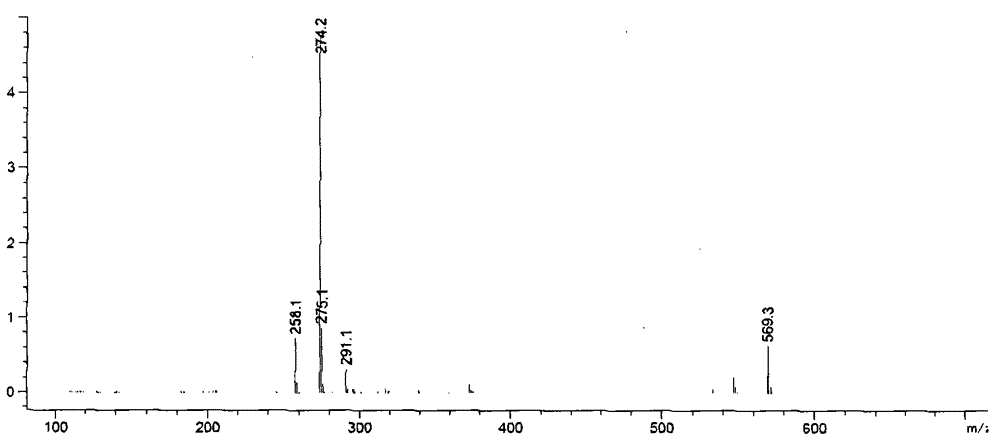
Preparation method of impurity compound in ramelteon and prepared standard substance
ActiveCN104402848BEasy to operateRaw materials are easy to getOrganic chemistryRetention timeLength wave
Owner:ZHUHAI UNITED LAB
Hydrocarbon Pyrolysis
ActiveUS20190276748A1Moderate yieldEasy to purifyThermal non-catalytic crackingCatalytic crackingPhysical chemistryPyrolysis
The invention relates to hydrocarbon pyrolysis, to equipment and materials useful for hydrocarbon pyrolysis, to processes for carrying out hydrocarbon pyrolysis, and to the use of hydrocarbon pyrolysis for, e.g., hydrocarbon upgrading.
Owner:EXXONMOBIL CHEM PAT INC
1,1-diacetate synthesis catalyzed by sulfonated cage-type mesoporous carbon
InactiveCN103319344BShort reaction timeHigh yieldOrganic compound preparationCarboxylic acid esters preparationChromatographic separationRecyclable catalyst
The invention relates to 1,1-diacetate synthesis catalyzed by sulfonated cage-type mesoporous carbon. The method is characterized in that aldehyde and acetic anhydride are placed in a reactor according to a molar ratio of 1:1-3; a sulfonated cage-type mesoporous carbon catalyst is added, wherein 5-20mg of the sulfonated cage-type mesoporous carbon catalyst is added to each mole of aldehyde; the materials are stirred under room temperature until a reaction is sufficiently carried out; ethyl acetate with a volume 20-30 times that of the reaction liquid is added; the catalyst is removed by filtering; a filtrate is washed three times by using a saturated NaHCO3 water solution, and once by using water; an organic phase is dried by using anhydrous Na2SO4; the solvent is removed by reduced-pressure distillation under a pressure of 0.09MPa, such that a crude product is obtained; and re-crystallization or column chromatographic separation is carried out, such that 1,1-diacetate is obtained. Compared with existing 1,1-diacetate synthesizing method, the method provided by the invention has the advantages of short reaction time, high yield, mild reaction condition, recyclable catalyst, and the like. The method can be used for synthesizing 1,1-diacetate.
Owner:SHAANXI NORMAL UNIV
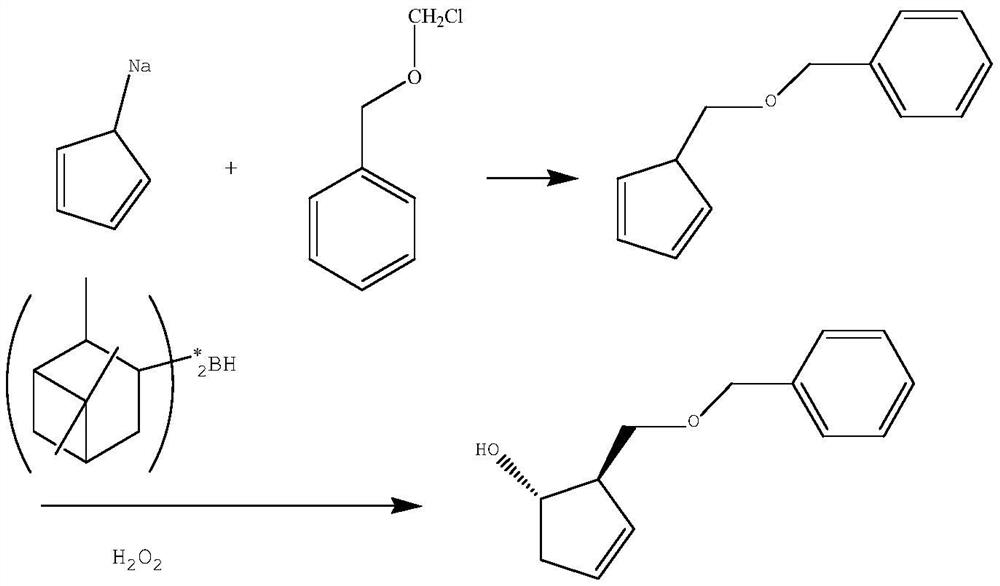
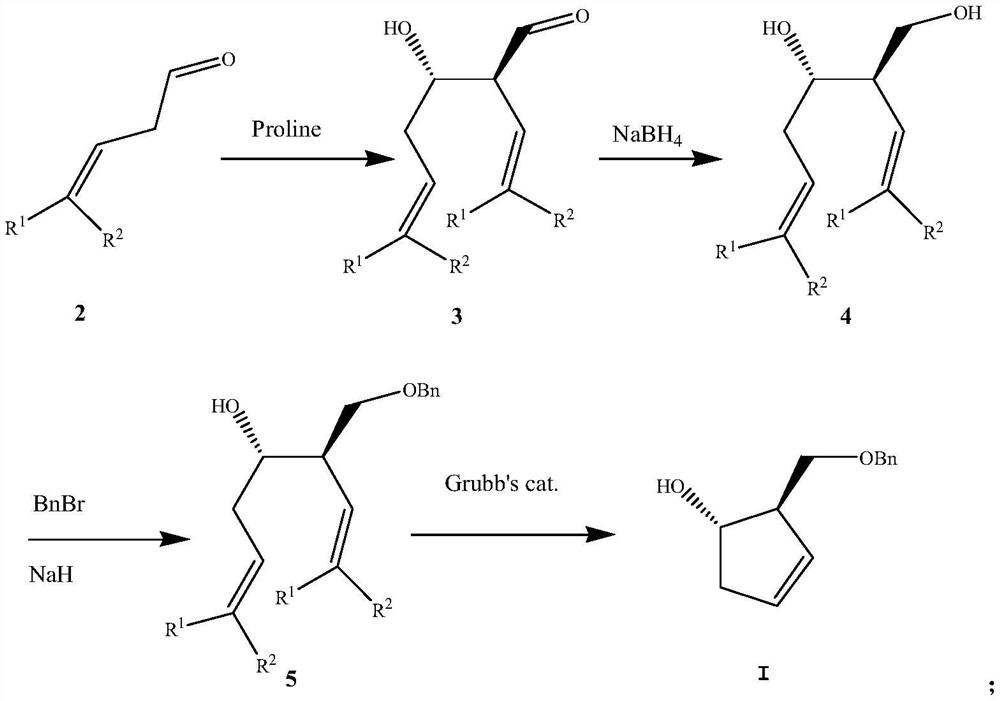
A kind of preparation method of pharmaceutical intermediate optically pure cyclopentenol
ActiveCN107188786BMild reaction conditionsEasy to operateOrganic compound preparationOrganic chemistry methodsCyclopenteneBenzoyl bromide
The present invention relates to a kind of preparation method of optically pure cyclopentenol of pharmaceutical intermediate, and this method is to use 3-butenal and its 3-position or 4-position derivative as raw material, in catalytic amount (S)-proline Synthesized in the presence of optically pure aldol condensation products, the aldehyde group was reduced to a hydroxyl group and then protected with benzyl bromide, and then obtained through a high-efficiency double bond metathesis ring-closure reaction (1 S ,2 R )-2-benzyloxymethylcyclopent-3-en-1-alcohol, the method provided by the invention has mild reaction conditions, cheap raw materials and reagents, high yield, and is suitable for industrial production.
Owner:HUBEI GRAND LIFE SCI & TECH CO LTD
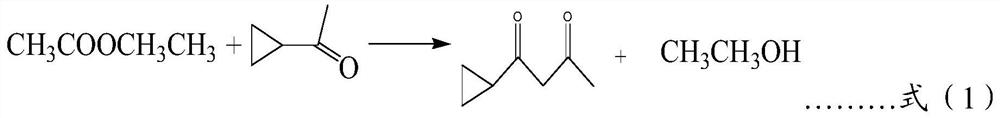
A kind of cyclopropionyl acetone and its synthetic method
ActiveCN108840791BGood unit costLow costOrganic compound preparationCarbonyl compound preparation by condensationKetoneEthyl acetate
The invention discloses a cyclopropionyl acetone and a synthesis method thereof, which comprises the following steps: adding sodium to absolute ethanol while stirring, lowering the temperature, reacting, and recovering ethanol to obtain a mixed solution; adding cyclopropylmethyl to the mixed solution Base ketone, stir, add 1 / 3 mass of ethyl acetate, reflux reaction, reclaim ethanol; then lower the temperature, add 1 / 3 mass of ethyl acetate, reflux reaction, reclaim ethanol; cool down again, add 1 / 3 mass of acetic acid Ethyl ester, reflux reaction, recovery of ethanol and ethyl acetate under negative pressure, cooling and storage, to obtain cyclopropyl methyl ketone sodium salt solution; adjust the pH of cyclopropyl methyl ketone sodium salt solution to 2~3, stir, and let stand , Take the upper layer of brownish-red liquid, and fractionally distill it to obtain cyclopropionyl acetone. The content of the obtained cyclopropionylacetone is about 90%, and the yield is greater than 90%. The synthesis method has mild reaction conditions, high catalytic efficiency, low energy consumption, low cost, easy recovery of the product, good safety, and is suitable for large-scale production of enterprises. .
Owner:陕西恒润化学工业有限公司
Improved method for preparing penfuridol
PendingCN111196781AEasy to operateModerate yieldOrganic chemistryBulk chemical productionMethyl benzeneSulfonic acid
The invention discloses an improved method for preparing penfuridol, comprising the following steps: using 4, 4-bis (4-fluorophenyl) butyric acid as a starting material, carrying out reduction synthesis to synthesize an intermediate I [4, 4-bis (4-fluorophenyl)-1-butanol]; carrying out esterification with a sulfonyl chloride compound to synthesize an intermediate II [4, 4-bis (4-fluorophenyl)-1 (4-methyl-phenyl)-butyl sulfonate] or [4, 4-bis (4-fluorophenyl)-1-methyl-butyl sulfonate]; and condensing with pipradrol to obtain penfuridol. According to the preparation method, 4, 4-bis (4-fluorophenyl) butyric acid is used as a raw material, penfuridol is obtained through a reduction reaction, an esterification reaction and a condensation reaction, the total yield is 81.3%, the whole process isconvenient to operate and mild in reaction, the obtained product is high in yield and purity, and the preparation method is more economical and more suitable for industrial application. The technicalproblems that an existing method is low in yield and poor in purity are solved. The reaction general formula is as shown in the specification.
Owner:湖南中南制药有限责任公司
Synthesis method of 1H-pyrrolo[2,3-b]pyridine-2-boronic acid pinacol ester
InactiveCN112159423AShort stepsCheap and easy to getGroup 3/13 element organic compoundsBulk chemical productionDicarbonateCarboxylic acid
The invention discloses a synthesis method of 1H-pyrrolo[2,3-b]pyridine-2-boronic acid pinacol ester, and belongs to the field of medicinal chemistry. The synthesis method comprises the following steps: reacting 7-azaindole serving as a raw material with di-tert-butyl dicarbonate to synthesize 1H-pyrrolo[2,3b]pyridine-1-carboxylic acid tert-butyl ester, reacting the 1H-pyrrolo[2,3b]pyridine-1-carboxylic acid tert-butyl ester with n-butyl lithium and 1,2-dibromotetrafluoroethane at low temperature to synthesize 2-bromo-1H-pyrrolo[2,3-b]pyridine, synthesizing 2-bromo-1H-pyrrolo[2,3-b]pyridine-1-carboxylic acid tert-butyl ester, removing the protective group to obtain 2-bromo-1H-pyrrolo[2,3-b]pyridine, and reacting 2-bromo-1H-pyrrolo[2,3-b]pyridine with bi-pinacol borate under the catalytic action of transition metals to obtain the target product. The raw materials and reagents are cheap and easy to obtain, reaction conditions are mild, the safety is high, the cost is low, and the preparation method is easily applied to industrial batch production.
Owner:凯美克(上海)医药科技有限公司
A high -grade tree alkaline compound and its synthesis method
The invention relates to camptothecin, in particular relates to homocamptothecin compounds and a synthesis method thereof, and mainly solves the technical problems of poor solubility and bioavailability of homocamptothecin in the prior art. Structural formulas of the homocamptothecin compounds are as shown in I and II, and the homocamptothecin compounds comprise racemic compounds and (S)-and (R)-stereisomers corresponding to the racemic compounds. The invention specifically relates to derivation of 10-hydroxyl homocamptothecin compound, the 10-hydroxyl homocamptothecin is used as a mother nucleus for synthesis of a series of homocamptothecin ester and amide compounds. The invention provides a new synthetic route, and according to the path, a full synthesis ring enlargement method is not needed, and protection and deprotection are not needed.
Owner:SUNDIA MEDITECH COMPANY LTD
Ceramide analog a and its preparation method and application
ActiveCN109627189BSynthetic conditions are mildEasy to controlUrea derivatives preparationOrganic compound preparationCancer cellLeukemia
The invention discloses a novel ceramide analogue A and a preparation method and application thereof, and belongs to the technical field of medicaments. The novel ceramide analogue structure introduces two amino groups to improve the carbonyl electronegativity between the two amino groups, and halogen is introduced at the tail end, so that the novel ceramide analogue is easier to combine with enzyme; the novel ceramide analogue has cis-double bonds, the solubility of molecules is improved, and the space trend of long chain connected with the double bonds is changed; the novel ceramide analoguecan not only inhibit the proliferation of tumor cells, but also have obvious influence on the differentiation and cycle of leukemia tumor cells, thereby being beneficial to multi-way tumor prevention; the novel ceramide analogue shows better performance in inhibiting human colorectal adenocarcinoma cell LS174T than positive control C6, shows a leader molecule with antitumor application potentialand has certain proliferation promoting effect on cancer cells.
Owner:ZHEJIANG UNIV
A New Method for Selective Oxidation of Alcohols Catalyzed by Nano-nickel Oxide
InactiveCN103288608BSimple preparation processLow priceOrganic compound preparationCarbonyl compound preparation by oxidationNitro compoundAlcohol
The invention relates to a novel method for catalyzing selective oxidization of an alcohol substance by using nanometer nickel oxide. The method disclosed by the invention comprises the steps of: with nanometer nickel oxide as a catalyst, a nitro-compound as an oxidizing agent and KOH as a synergist, catalyzing and oxidizing an alcohol compound so as to prepare aldehyde or ketone at a gage pressure of 0-1 MPa and at a reaction temperature of 50-150 DEG C, and a corresponding nitro-compound is reduced into amine. According to the method disclosed by the invention, the defects that a catalyst is expensive, unsafe and not environment-friendly, and has high requirements on equipment, etc are overcome. The method disclosed by the invention can be used for simultaneously catalyzing selective oxidization of the alcohol compound and selective reduction of the nitro-compound; a preparation process of the needed catalyst is simple; the catalyst is cheap; the novel method can be operated at low temperature and normal pressure when being applied to the reaction and are mild in condition, high in selectivity and moderate in yield.
Owner:YANGZHOU UNIV
Methods of preparing secondary carbinamine compounds with boronic acids
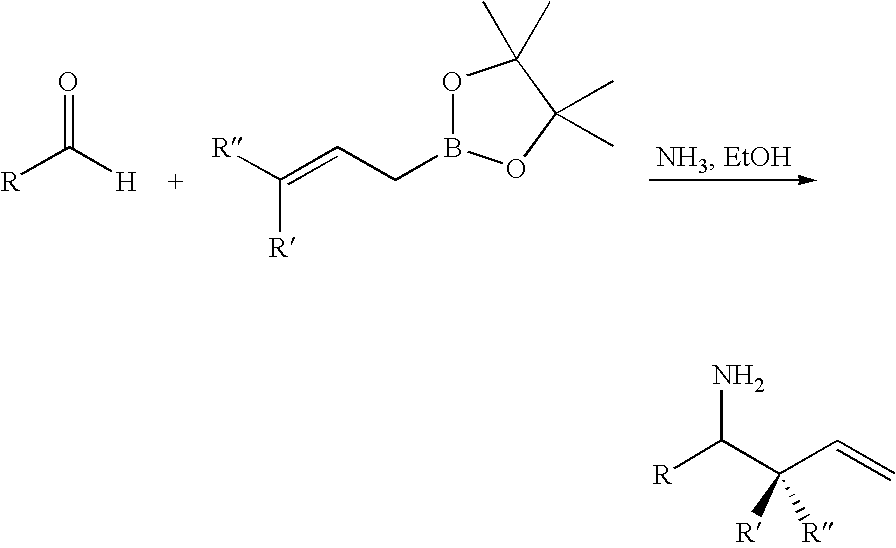
InactiveUS20100298572A1Moderate yieldSimple and efficientAmino compound purification/separationCarboxylic acid nitrile preparationBoronic acidCombinatorial chemistry
The present application relates to novel methods for the preparation of secondary carbinamine compounds, particularly the preparation of secondary carbinamine compounds of the formula Ia, formula Ib or formula IV from aldehydes of the formula II and boronic acids of the formula III or formula V, in the presence of ammonia or an ammonia equivalent of the formula NH4+X−.
Owner:PANASONIC CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com

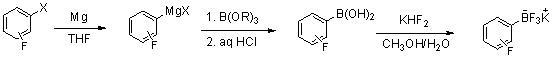



![Synthesis method of tert-butyl-1, 7-diazaspiro [3.5] nonane-1-formate Synthesis method of tert-butyl-1, 7-diazaspiro [3.5] nonane-1-formate](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/c419c075-256f-4485-9366-6c39e9d8594c/204216DEST_PATH_IMAGE004.png)
![Synthesis method of tert-butyl-1, 7-diazaspiro [3.5] nonane-1-formate Synthesis method of tert-butyl-1, 7-diazaspiro [3.5] nonane-1-formate](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/c419c075-256f-4485-9366-6c39e9d8594c/349392DEST_PATH_IMAGE002.png)


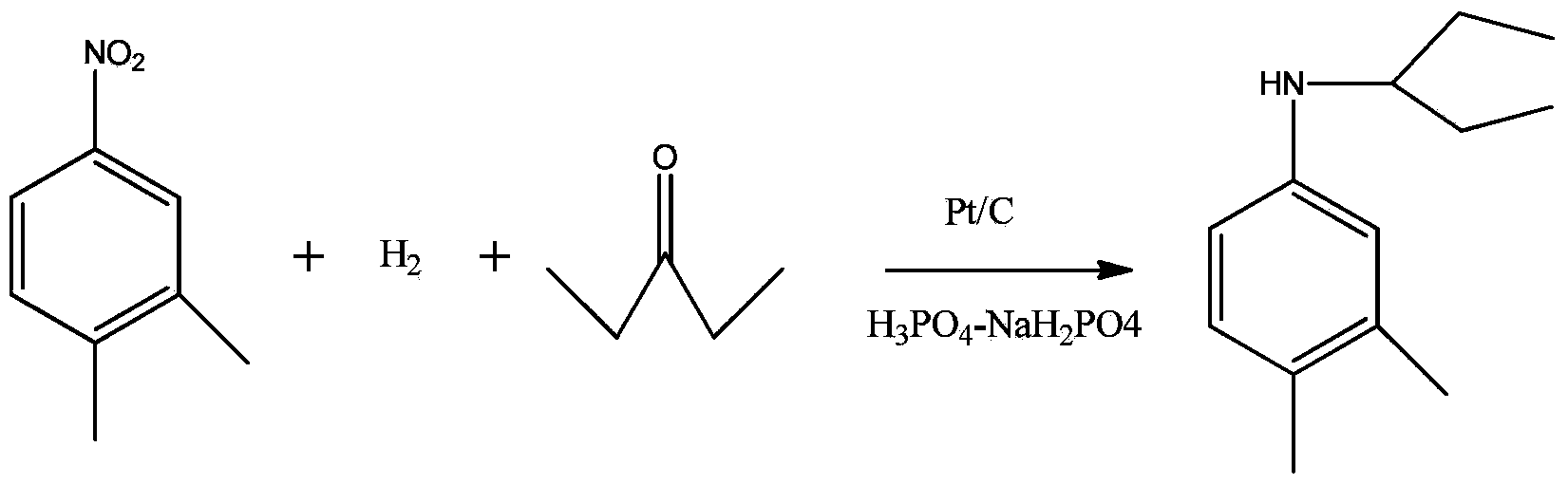



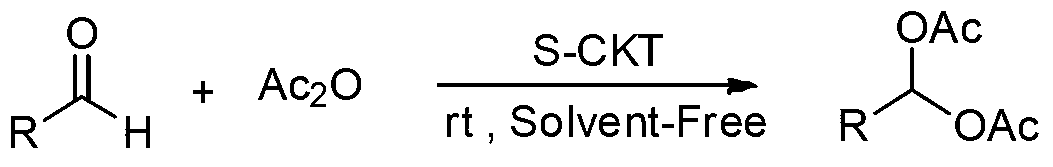









![A kind of synthetic method of polysubstituted benzo [c, d] indole compound A kind of synthetic method of polysubstituted benzo [c, d] indole compound](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/000be05c-0415-44a4-a1e9-62fdeeee68e8/BDA0001524740520000031.png)
![A kind of synthetic method of polysubstituted benzo [c, d] indole compound A kind of synthetic method of polysubstituted benzo [c, d] indole compound](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/000be05c-0415-44a4-a1e9-62fdeeee68e8/BDA0001524740520000041.png)











![Synthesis method of 1H-pyrrolo[2,3-b]pyridine-2-boronic acid pinacol ester Synthesis method of 1H-pyrrolo[2,3-b]pyridine-2-boronic acid pinacol ester](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/424e69f3-6d8b-4524-b5fc-9b76cda70c43/HDA0002758107320000011.png)
![Synthesis method of 1H-pyrrolo[2,3-b]pyridine-2-boronic acid pinacol ester Synthesis method of 1H-pyrrolo[2,3-b]pyridine-2-boronic acid pinacol ester](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/424e69f3-6d8b-4524-b5fc-9b76cda70c43/HDA0002758107320000012.png)
![Synthesis method of 1H-pyrrolo[2,3-b]pyridine-2-boronic acid pinacol ester Synthesis method of 1H-pyrrolo[2,3-b]pyridine-2-boronic acid pinacol ester](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/424e69f3-6d8b-4524-b5fc-9b76cda70c43/FDA0002758107300000011.png)