Preparation method of palbociclib for treating breast cancer
A breast cancer and compound technology, applied in the field of drug synthesis, can solve the problems of long reaction time, low product yield, harsh conditions, etc., and achieve the effect of simple operation steps, increased yield, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] A preparation method for palbociclib for treating breast cancer, comprising the following steps:
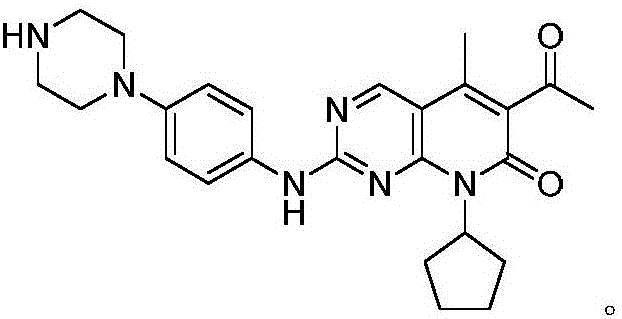
[0032] 1) Under the protection of nitrogen, the compound N-cyclopentyl-2-methoxy-3-cyano-4-methyl-5-acetyl-6-oxopiperidine-2 shown in formula (I) -ene 27.6g (100mmol), compound N-[5-(1-piperazinyl)-2-pyridyl]guanidine represented by formula (II) 35.2g (160mmol), compound 20.5 represented by formula (M) g (60mmol) was added to 300ml 1,4-dioxane for 6 hours of contact reaction, the temperature of the contact reaction was 95°C, after the reaction was completed, the solvent was evaporated under reduced pressure, washed with water, recrystallized from methanol, and dried to obtain Compound 6-acetyl-8-cyclopentyl-5-methyl-2-[[5-(1-piperazinyl)-2-pyridyl]amino]-5,6- Dihydropyrido[2,3-d]pyrimidin-7(8H)-one 37.6g, yield 83.7%, purity 99.82% (HPLC area normalization method).
[0033] 2) Under nitrogen protection, the 6-acetyl-8-cyclopentyl-5-methyl-2-[[5-(1-piperazinyl)-2-pyridyl]...
Embodiment 2
[0036] A preparation method for palbociclib for treating breast cancer, comprising the following steps:
[0037]1) Under the protection of nitrogen, the compound N-cyclopentyl-2-methoxy-3-cyano-4-methyl-5-acetyl-6-oxopiperidine-2 shown in formula (I) -ene 27.6g (100mmol), compound N-[5-(1-piperazinyl)-2-pyridyl]guanidine represented by formula (II) 41.1g (200mmol), compound 27.3 represented by formula (M) g (80mmol) was added to 300ml 1,4-dioxane for 6 hours of contact reaction, the temperature of the contact reaction was 90°C, after the reaction was completed, the solvent was evaporated under reduced pressure, washed with water, recrystallized from methanol, and dried to obtain Compound 6-acetyl-8-cyclopentyl-5-methyl-2-[[5-(1-piperazinyl)-2-pyridyl]amino]-5,6- Dihydropyrido[2,3-d]pyrimidin-7(8H)-one 37.5g, yield 83.5%, purity 99.82% (HPLC area normalization method).
[0038] 2) Under nitrogen protection, the 6-acetyl-8-cyclopentyl-5-methyl-2-[[5-(1-piperazinyl)-2-pyridyl]a...
Embodiment 3
[0041] A preparation method for palbociclib for treating breast cancer, comprising the following steps:
[0042] 1) Under the protection of nitrogen, the compound N-cyclopentyl-2-methoxy-3-cyano-4-methyl-5-acetyl-6-oxopiperidine-2 shown in formula (I) -ene 27.6g (100mmol), compound N-[5-(1-piperazinyl)-2-pyridyl]guanidine represented by formula (II) 39.7g (180mmol), compound represented by formula (M) 17g (50mmol) was added into 300ml 1,4-dioxane and carried out contact reaction for 7.5 hours. The temperature of the contact reaction was 85°C. After the reaction was completed, the solvent was evaporated under reduced pressure, washed with water, recrystallized from methanol, and dried to obtain the formula The compound shown in (III) 6-acetyl-8-cyclopentyl-5-methyl-2-[[5-(1-piperazinyl)-2-pyridyl]amino]-5,6-di Hydropyrido[2,3-d]pyrimidin-7(8H)-one 37.3g, yield 82.9%, purity 99.82% (HPLC area normalization method).
[0043] 2) Under nitrogen protection, the 6-acetyl-8-cyclopen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com