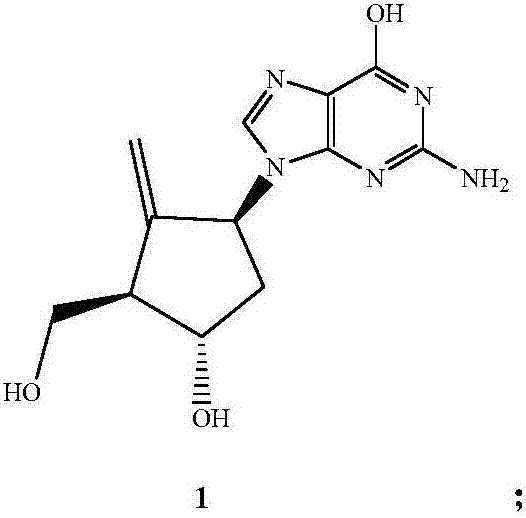
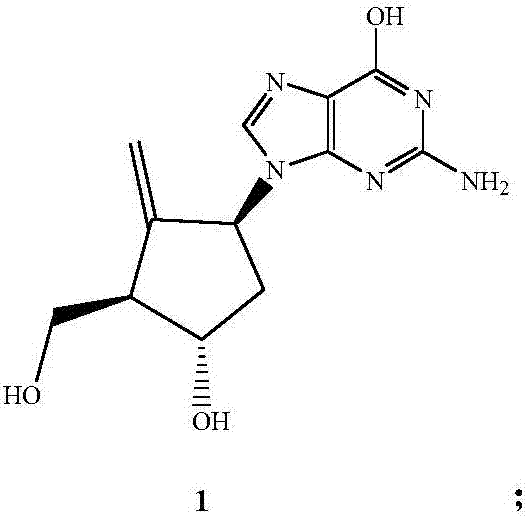
Preparation method of entecavir
A technology of entecavir and compound, which is applied in the field of preparation of entecavir to achieve the effects of mild reaction conditions, high purity and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1 Preparation of (3aS, 6aR)-5-formaldehyde-3,3a,6,6a-tetrahydro-2H-cyclopenta[b]furan-2-ol
[0037] Succindialdehyde (5.75 g, 66.8 mmol) was stirred in tetrahydrofuran (130 ml) at 25°C, (R)-proline (150 mg, 1.3 mmol) was added at one time, stirred at 25°C for 40h, and then di Benzylamine trifluoroacetate (416 mg, 1.34 mmol). Stirring was continued at 25 °C for 20 h. After the reaction solution was concentrated to 20 ml, 30 ml of methyl tert-butyl ether was added, filtered, and the filtrate was concentrated to obtain (3aR,6aS)-5-formaldehyde-3,3a,6,6a-tetrahydro-2H-cyclopenta [b] The crude product of furan-2-ol was directly used in the next reaction.
Embodiment 2
[0038] Example 2 Preparation of (3aS, 6aR)-5-formaldehyde-3,3a,6,6a-tetrahydro-2H-cyclopenta[b]furan-2-ol (2)
[0039] The crude product obtained above was dissolved in 12 ml of dichloromethane, methanol (340 mg), ion exchange resin 15 (76 mg) and magnesium sulfate (1.6 g) were added at 25 °C, stirred at 25 °C for 20 h, and the reaction solution was filtered Concentration and column chromatography gave 2.10 g of (3aS,6aR)-5-formaldehyde-3,3a,6,6a-tetrahydro-2H-cyclopenta[b]furan-2-ol (2). The two-step total yield is 35%, and the proton magnetic spectrum is consistent with the literature.
Embodiment 3
[0040] Example 3 Preparation of (3aS, 6aR)-2-methoxyl-3,3a,6,6a-tetrahydro-2H-cyclopenta[b]furan
[0041] Mix 180 mg of (3aR,6aS)-2-methoxy-5-formyl-3,3a,6,6a-tetrahydro-2H-cyclopenta[b]furan (1.0 mmol) with tris(triphenyl Phosphine) rhodium chloride (0.8 g, 0.8 mmol) was added to benzonitrile (5 ml) respectively, heated to 130° C., kept for 1.5 hours, added ether after cooling in an ice bath, filtered, the solid was washed with ether, and combined The extract was dried, concentrated, and purified by column chromatography to obtain (3aS,6aR)-2-methoxy-3,3a,6,6a-tetrahydro-2H-cyclopenta[b]furan 129 mg, yield 85%, 1 H NMR (DMSO-d 6 )δ: 1.95 (brs, 2H), 2.41 (m, 2H), 2.58 (m, 1H), 3.24 (m, 1H), 3.27 (s, 3H), 5.10 (m, 1H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com