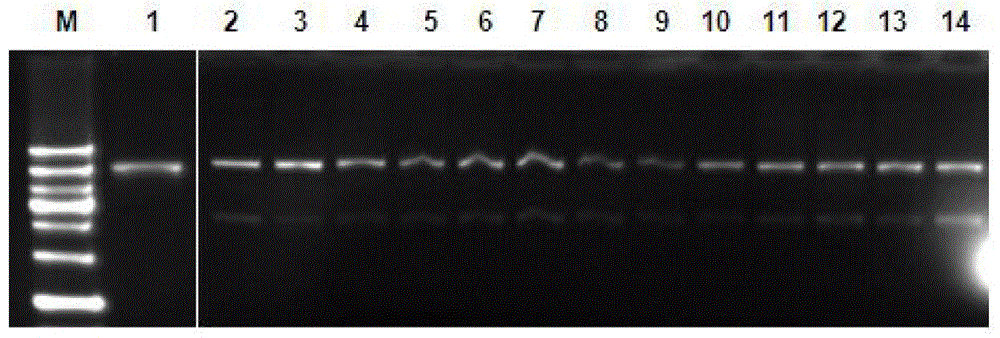
Homocamptothecin compound and synthesis method thereof
A technology of homocamptothecin and its synthesis method, which is applied in the field of camptothecin and can solve the problems of poor solubility and bioavailability of homocamptothecin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Embodiment 1: ( S Synthesis of )-10-((dibutoxyphosphate)oxo)camptothecin (2)
[0040] Under an ice bath, slowly add elemental iodine (75.0 g, 0.30 mol) to a dichloromethane solution (200 mL) dissolved with tributyl phosphite (94.0 g, 0.38 mol), and the reaction system was maintained at this temperature Stir for half an hour, then remove the ice bath and gradually warm to room temperature. To the above reaction system, slowly dropwise add 10-hydroxycamptothecin (1,50.0 g, 0.14 mol) and 4-dimethylaminopyridine (42.0 g, 0.34 mol) in dichloromethane solution (600 mL) . The reactant was stirred at room temperature overnight. The organic phase was washed successively with 1M hydrochloric acid (200 mL x3), brine (200 mL x3), dried over anhydrous sodium sulfate, and concentrated to dryness. The residue was subjected to column chromatography (DCM:CH 3 OH=200:1-60:1) purified to obtain 47.0 g (yield: 60%) ( S )-10-((dibutoxyphosphate)oxo)camptothecin (1), as a yellow solid. ...
Embodiment 2
[0041] Example 2: Dibutyl (4-ethyl-3,4-dihydroxy-14-oxo-3,4,12,14-tetrahydro-1H-pyranoindole-o-[1,2-b Synthesis of ]quinolin-9-yl)phosphate (3)
[0042] Xiang Rongyou ( S )-10-((dibutoxyphosphate) oxo)camptothecin (2, 47.0 g, 84.5 mmol) in anhydrous methanol solution (300 mL), under ice-cooling, within 10 minutes, add the solid Sodium borohydride (3.2 g, 84.5 mmol). The reaction was reacted at room temperature for half an hour, then quenched with saturated aqueous ammonium chloride (200 mL), and concentrated to dryness. The residue was dissolved in ethyl acetate (300 mL), then washed with saturated brine (100 mLx3), then dried over anhydrous sodium sulfate, and concentrated to dryness to obtain 37.7 g (yield: 80.0%) of dibutyl ( 4-Ethyl-3,4-dihydroxy-14-oxo-3,4,12,14-tetrahydro-1H-pyranoindole-o[1,2-b]quinolin-9-yl) Phosphate ester synthesis (3), was pale yellow solid.
Embodiment 3
[0043] Example 3: {3-[1-(4-chloro-benzyl)-3-(2-cyano-thiophen-3-yl)-1 H Synthesis of -indol-2-yl]-phenoxy}-acetic acid (4)
[0044] In an ice bath, dibutyl (4-ethyl-3,4-dihydroxy-14-oxo-3,4,12,14-tetrahydro-1H-pyranoindole-o-[1,2 -b] quinoline-9-yl) phosphate (3, 2.00 g, 3.58 mmol) in acetone (30 mL) suspension liquid, slowly dropwise add sodium periodate aqueous solution (1.5 g, 11.7 mmol, 6 mL) . After the dropwise addition, the reaction solution rose to 50 o C, stirred for 5 hours. The reaction solution was concentrated to dryness, and the residue was dissolved in ethyl acetate (50 mL), washed with saturated brine (30mLx3), dried over anhydrous sodium sulfate and concentrated to dryness. The crude product was purified by column chromatography (DCM: CH 3 OH=100:1-40:1), to obtain 1.50 g (yield: 75%) {3-[1-(4-chloro-benzyl)-3-(2-cyano-thiophen-3-yl) -1 H -Indol-2-yl]-phenoxy}-acetic acid (4) as pale yellow solid.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com