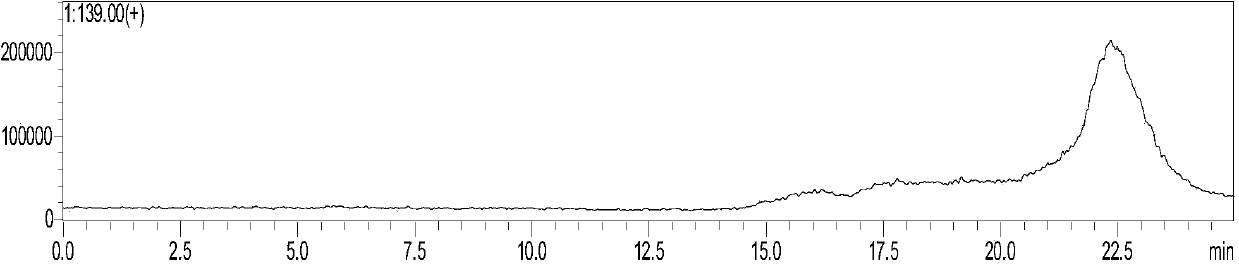
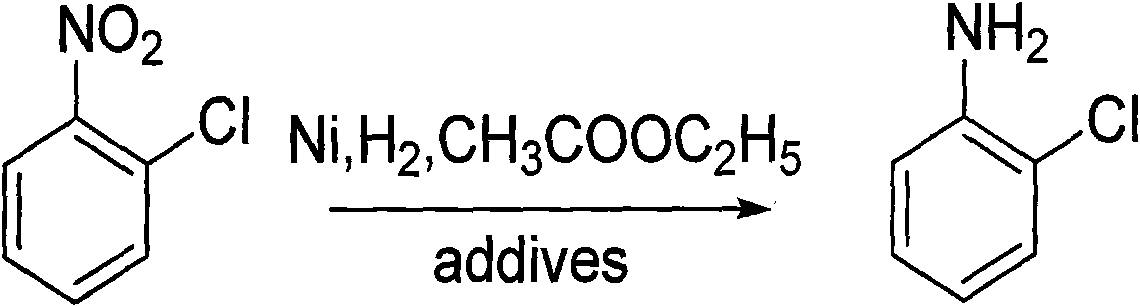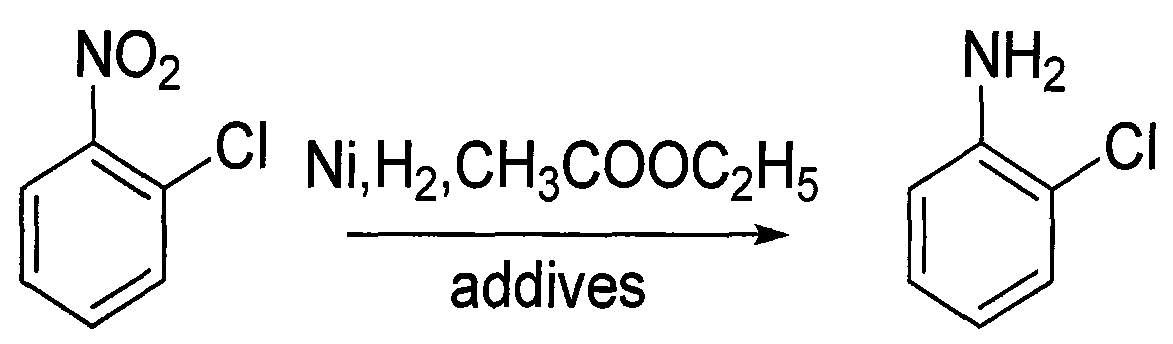Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
67 results about "O-chloroaniline" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
2-chloroaniline hydrochloride; o-chloroaniline; from MeSH. Depositor-Supplied Synonyms. Chemical names and identifiers provided by individual data contributors and associated to PubChem Substance records. Synonyms of Substances corresponding to a PubChem Compound record are combined.
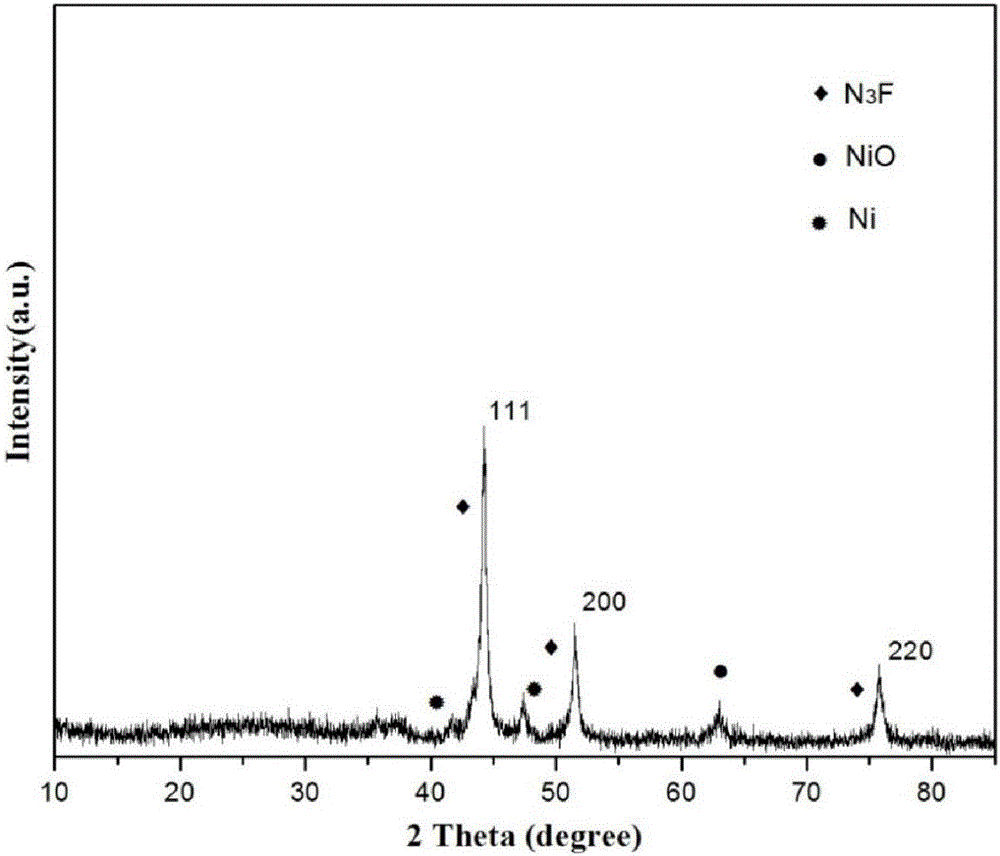
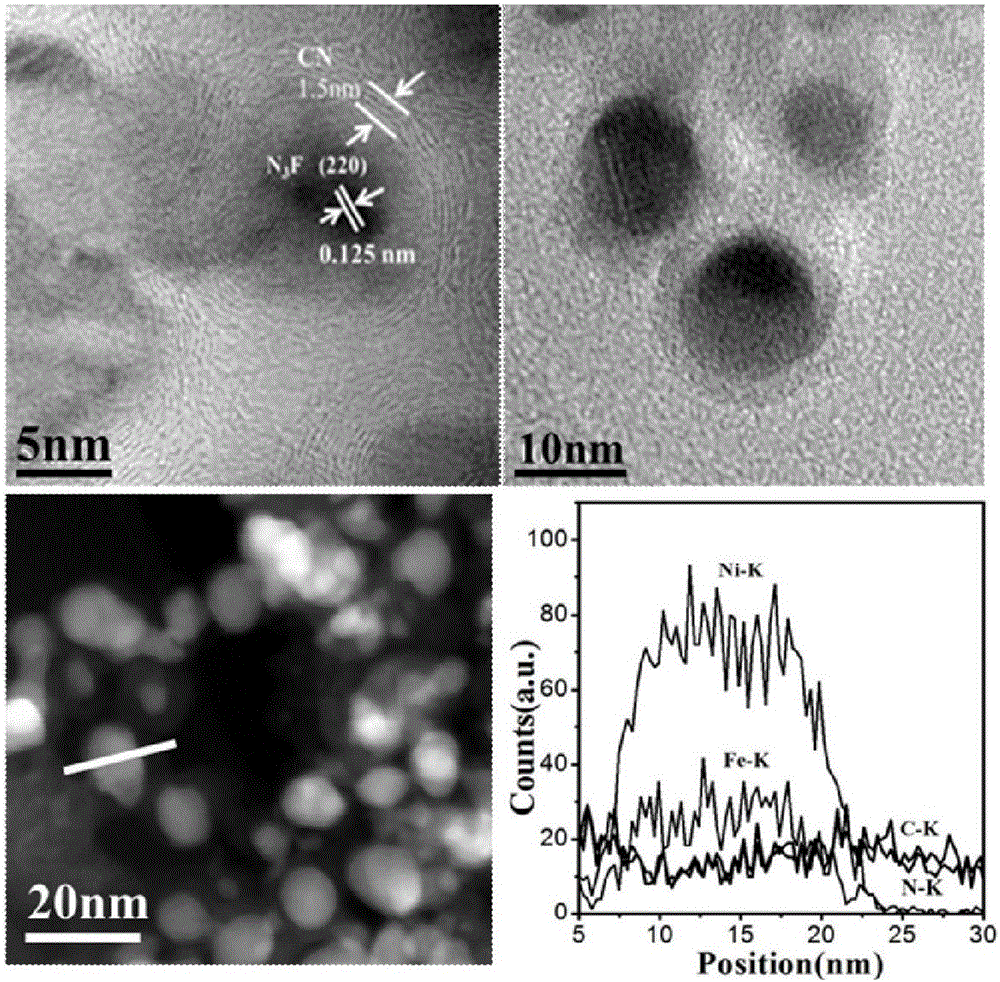
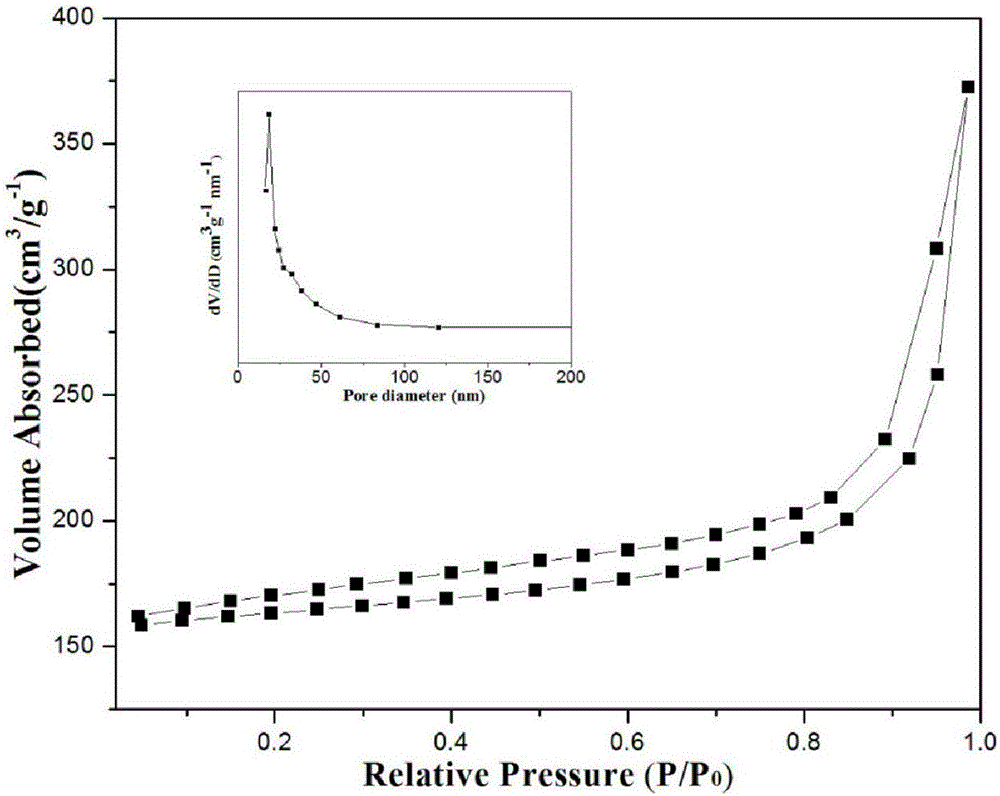
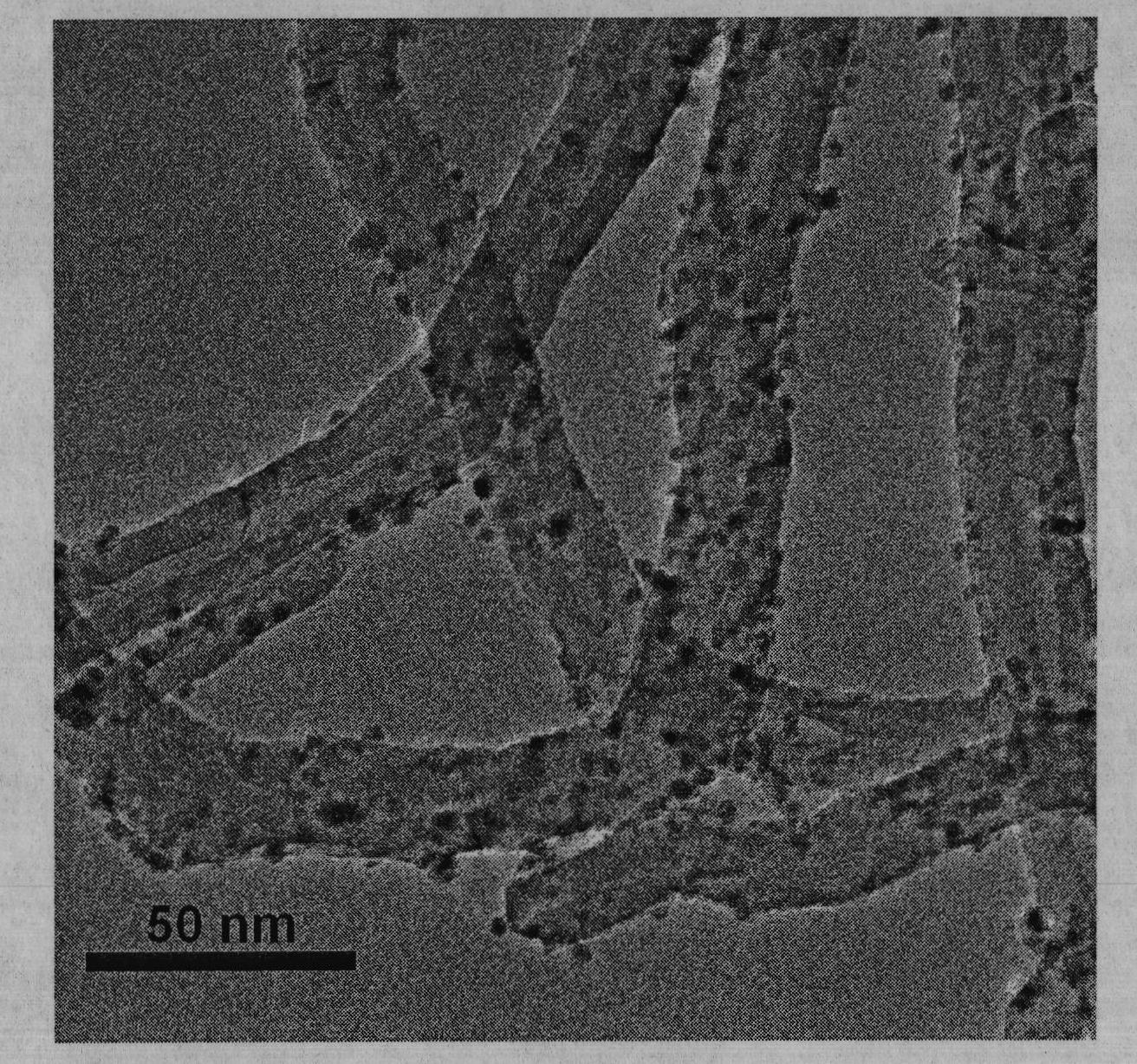
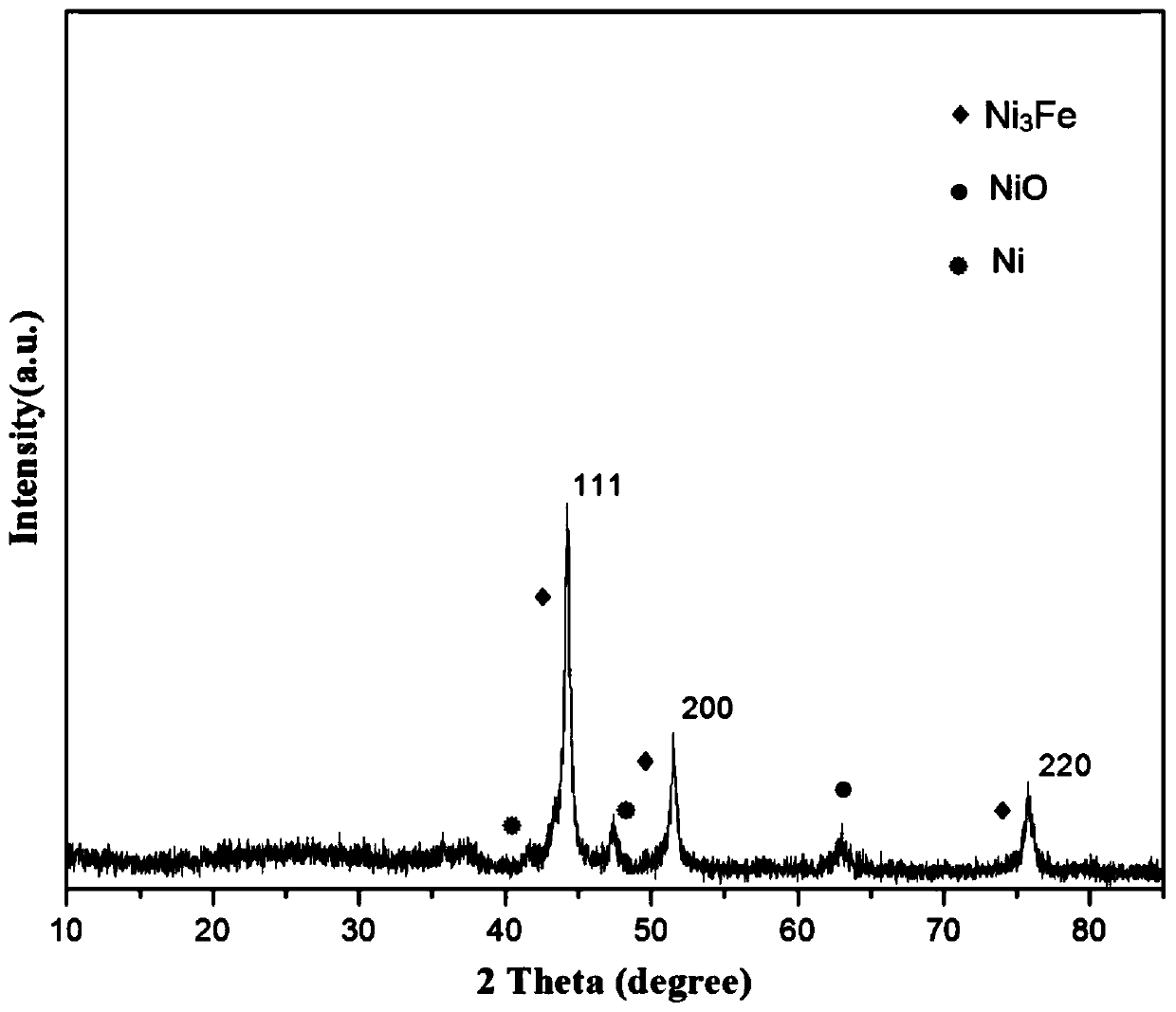
Preparation of nitrogen doped carbon-encapsulated core-shell structure ferro-nickel nano-catalyst and application thereof in catalyzing o-chloronitrobenzene hydrogenation reaction
ActiveCN106732733AMaterial nanotechnologyPhysical/chemical process catalystsNano catalystNitro compound
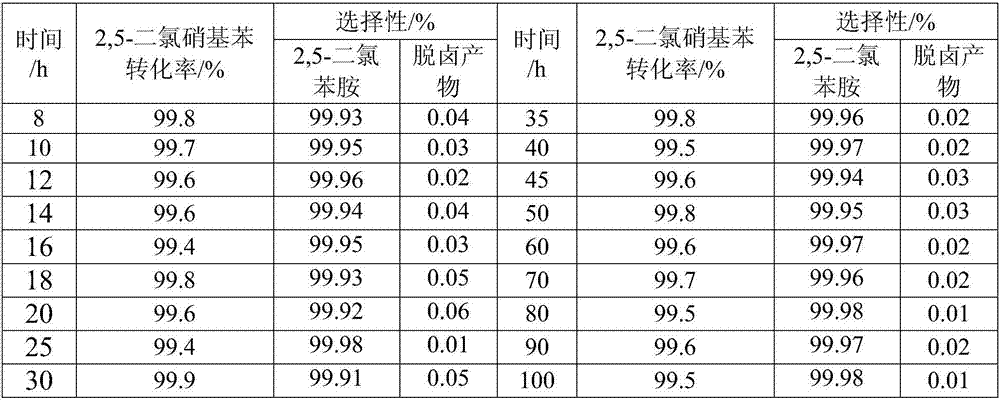
The invention provides a preparation method of a nitrogen doped carbon-encapsulated core-shell structure ferro-nickel nano-catalyst and the application of the nitrogen doped carbon-encapsulated core-shell structure ferro-nickel nano-catalyst in catalyzing an o-chloronitrobenzene hydrogenation reaction. According to the method, the novel nitrogen doped carbon-encapsulated core-shell structure ferro-nickel nano-catalyst is prepared by synthesizing a ferronickel layered doubled hydroxide precursor with small grain size and high surface energy through a nucleation crystallization isolation method, evenly mixing the ferronickel layered doubled hydroxide precursor with a melamine and dicyandiamide mixed carbon material precursor, and finally self-reducing at high temperature. The nitrogen doped carbon-encapsulated core-shell structure ferro-nickel nano-catalyst is efficiently applied to the reaction where halogenated aniline is generated through catalytic hydrogenation of a nitro-halogen compound, and the conversion rate of o-chloronitrobenzene and the selectivity of o-chloroaniline are respectively up to 95-100% and 98-100%. The structure of the novel nitrogen doped carbon-encapsulated core-shell structure ferro-nickel nano-catalyst is unique and novel, the process is green and energy-saving, the structure of the catalyst is stable, and the catalyst has a broad application prospect.
Owner:BEIJING UNIV OF CHEM TECH
Production method for preparing chlorinated aniline via chlorination of nitrobenzene hydrogenation by utilizing solvent-free process
InactiveCN103113233ALow dechlorinationReduce addOrganic compound preparationAmino compound preparationSolvent freeNitrobenzene
The invention belongs to the production field of catalytic hydrogenation, and particularly discloses a production method for preparing chlorinated aniline via the chlorination of nitrobenzene hydrogenation by utilizing a solvent-free process. Chlorinated nitrobenzene is used as a raw material. The method is characterized in that under the existence of a catalyst and an aiding agent, the chlorinated nitrobenzene reacts with hydrogen at the temperature of 80 to 100 DEG C and under the pressure of 0.3 to 2.5 MPa, wherein a solvent is not added; and after the reaction is finished, the water diversion treatment is carried out, thereby obtaining the chlorinated aniline. The catalyst containing 1% of Pt / C precious metal is developed independently, wherein the adding amount of the catalyst accounts for 0.05% to 20% of that of the chlorinated nitrobenzene as the raw material. The aiding agent is a mixture of ethanol amine and pyridine, wherein the adding amount of the aiding agent accounts for 0.01% to 10% of that of the chlorinated nitrobenzene as the raw material. For the obtained o-chloroaniline, the chromatographic purity is above 99.5%, and the dechlorination quantity can be controlled within 0.1%. For the obtained 2,5-dichloroaniline, the chromatographic purity is above 99%, and the dechlorination quantity can be controlled within 0.1%. For the obtained 3,4-dichloroaniline, the chromatographic purity is above 99%, and the dechlorination quantity can be controlled within 0.1%.
Owner:SHANDONG FUYUAN CHEM
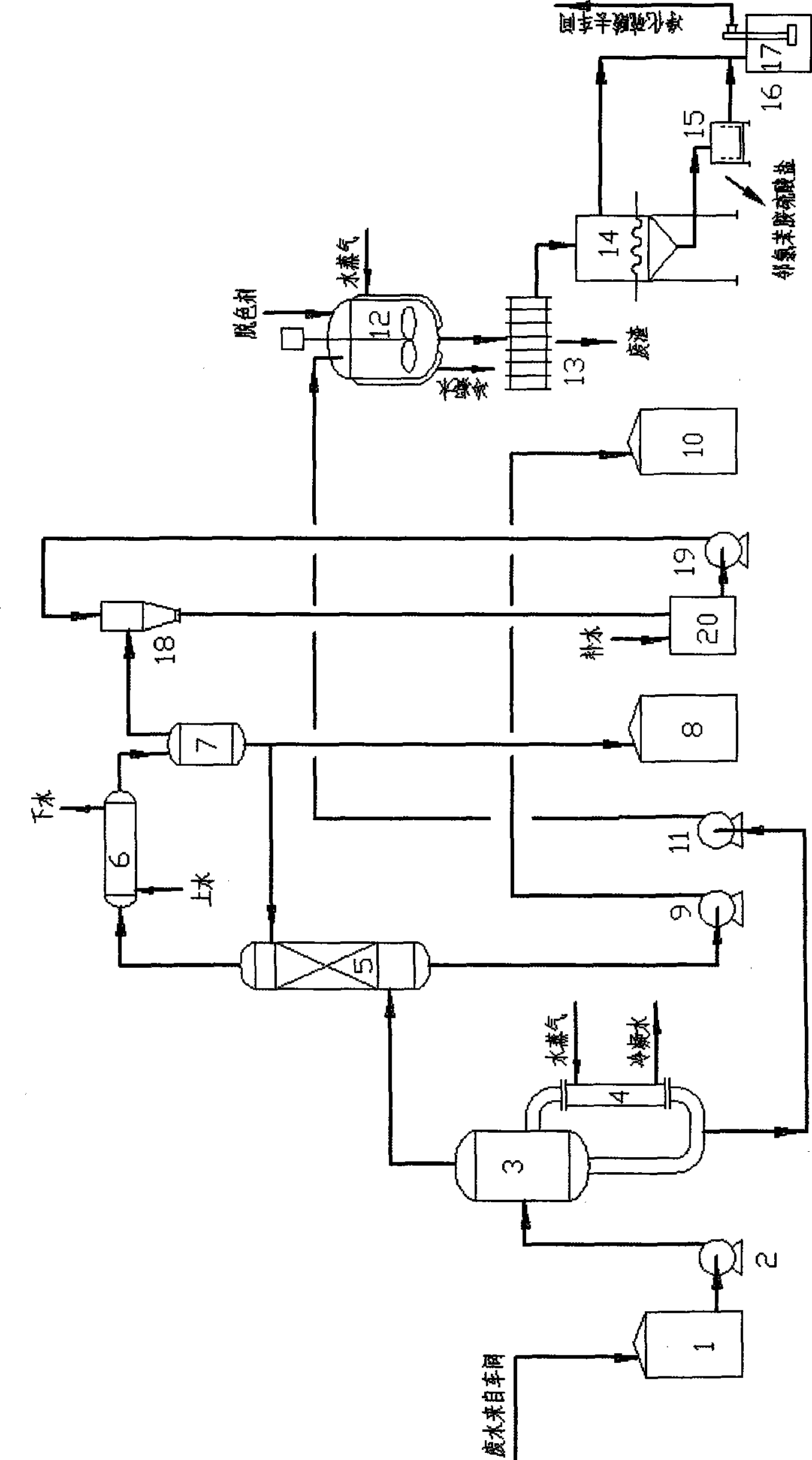
3,3'-dichlorobenzidine hydrochloride waste water reclaiming method and apparatus thereof
InactiveCN101372381AReduce wasteHigh purityMultistage water/sewage treatmentSulfur-trioxide/sulfuric-acidWater vaporPollution
The invention discloses a method for resourceful treatment of 3,3'-dichlorobenzidine hydrochloride wastewater, in which a chemical technology is adopted for the resourceful treatment of 3, 3'-dichlorobenzidine hydrochloride wastewater, in order that hydrochloric acid and sulphuric acid in the wastewater can be recycled, a by-product of o-chloroaniline sulfate is obtained, and the environment pollution caused by waste discharge is prevented. The method for resourceful treatment comprises that (1) first, the wastewater is evaporated, so that the chlorine hydride in the wastewater is separated and the wastewater is concentrated; (2) the chlorine hydride and water evaporated is rectified and separated, so that the chlorine hydride can be concentrated and reused, the vapor containing little chlorine hydride is recycled by condensing; (3) the wastewater concentrated by evaporating is decolored, and the colored substances contained are removed; (4) the wastewater is cooled for crystallization after being decolored, so that the o-chloroaniline sulfate is separated, and the purified sulphuric acid is obtained and reused. The invention discloses the structure of a device for resourceful treatment technique at the same time.
Owner:SHANDONG AGRICULTURAL UNIVERSITY
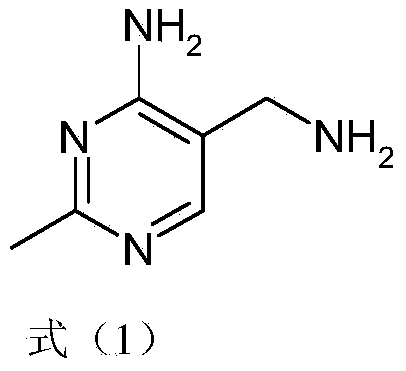
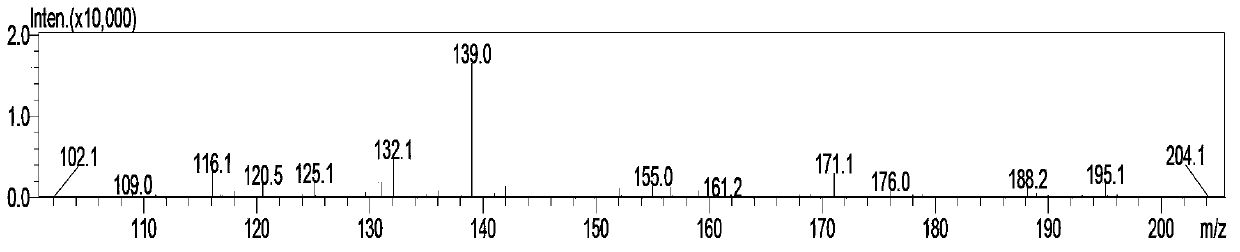
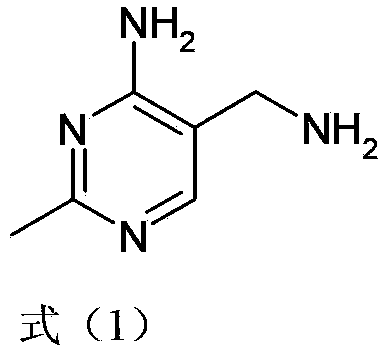
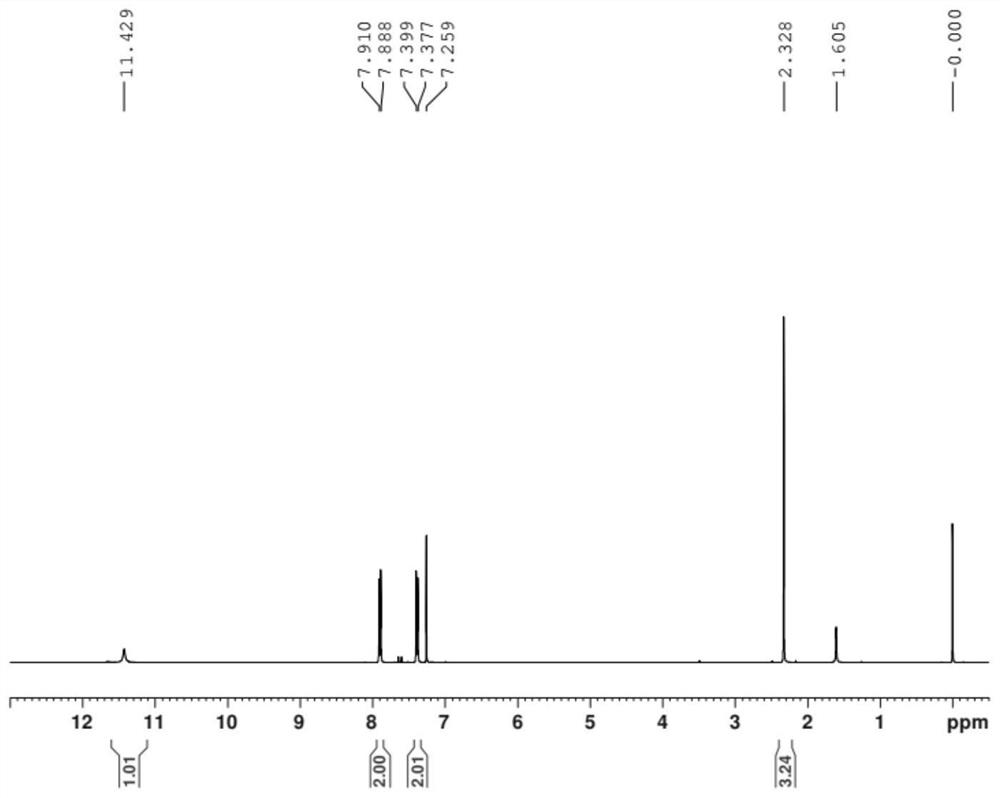
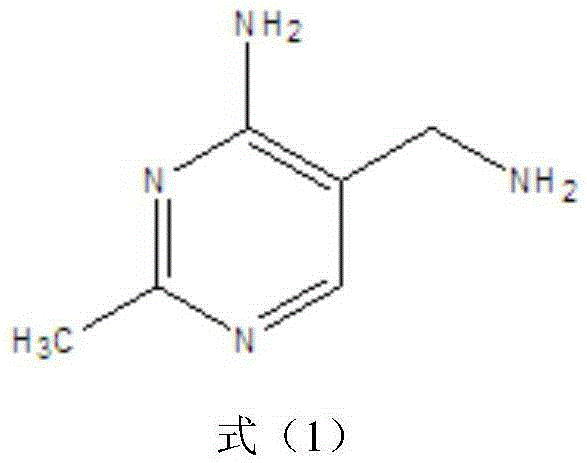
Simple and quick method for synthesizing improved vitamin B1 intermediate 2-methyl-4-amino-5-aminomethylpyrimidine
ActiveCN103435556AReduce concentrationReduce decompositionOrganic chemistryAminopropionitrilePropionitrile
The invention relates to a simple and quick method for synthesizing an improved vitamin B1 intermediate 2-methyl-4-amino-5-aminomethylpyrimidine. The method comprises the following steps: condensing 3-alkyl (aryl) formamido-propionitrile serving as a raw material with acetamidine in the catalytic action of lewis acid; cyclizing with trimethyl orthoformate, and hydrolyzing under an alkaline condition to prepare the vitamin B1 key intermediate 2-methyl-4-amino-5-aminomethylpyrimidine. The four reacting processes are performed in sequence by a one-pot reaction, and the product in each step does not need to be separated and purified. According to the method, highly carcinogenic o-chloroaniline or other small molecular aniline compounds are not used, residues of o-chloroaniline compounds in the vitamin B1 product can be eliminated. Furthermore, the preparation process is short and convenient in flow, small in wastewater amount and high in yield.
Owner:XINFA PHARMA
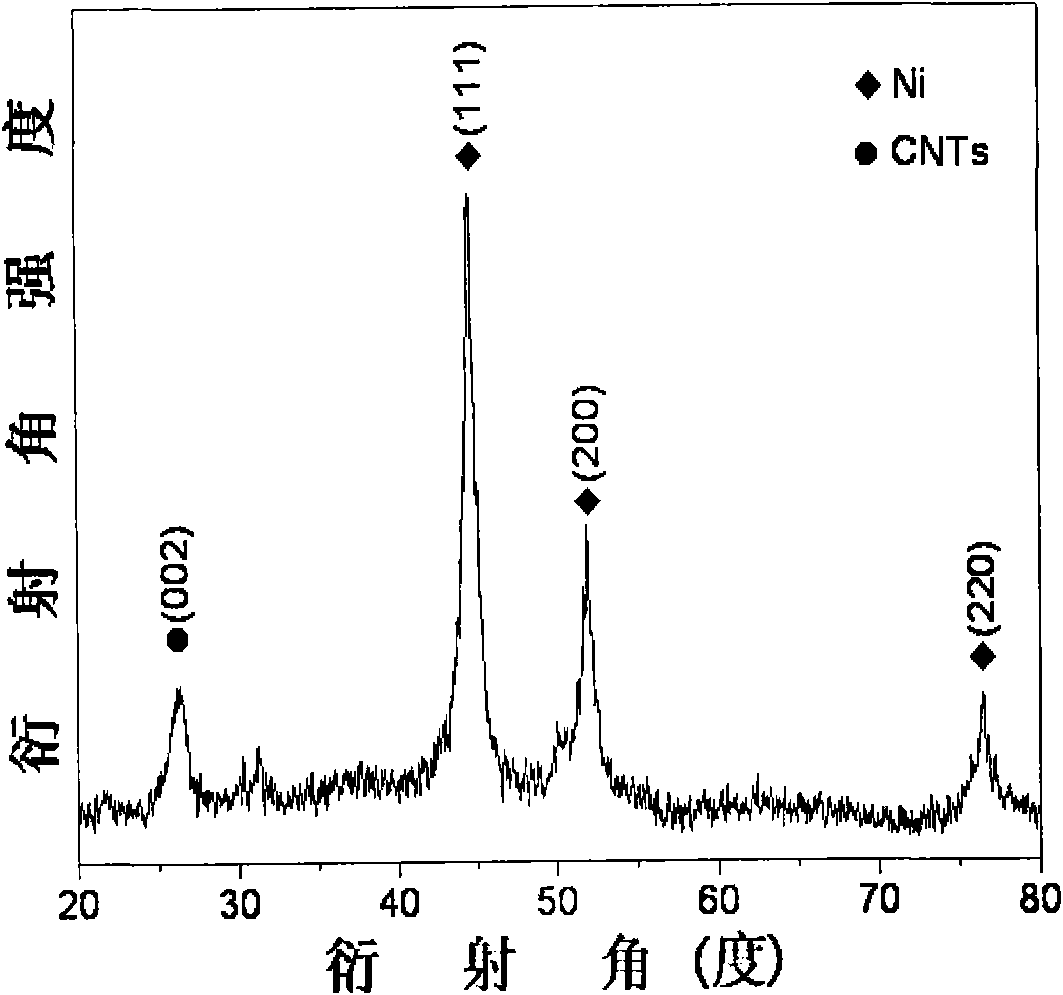
High dispersed loaded nano-metal Ni catalyst and preparation method thereof
InactiveCN102068991AFirmly assembledGuaranteed smooth assemblyMaterial nanotechnologyOrganic compound preparationCarbon nanotubeParticle-size distribution
The invention provides a high dispersed loaded nano-metal Ni catalyst and a preparation method thereof, and belongs to the technical field of preparation of metal nano-particles. The bridging role of L-cysteine is utilized, the co-precipitation method is adopted for firstly preparing a NiAl-layered double-metal hydroxide / polyacrylic acid surface functional carbon nano-tube complex precursor, and then the high dispersed Ni metal catalyst loaded by carbon nano-tubes is further obtained by reducing the precursor through hydrogen. The loaded nano-metal Ni catalyst is formed by uniformly loading the mixture of Ni nano-particles and amorphous Al2O3 on the surface of the carbon nano-tubes, wherein the weight percentage content of the Ni is 3-30%, the weight percentage content of the amorphous Al2O3 is 1-10%, and the weight percentage content of the carbon nano-tubes is 60-95%; and the particle size distribution of the Ni nano-particles is 6-12nm. The catalyst is applied in hydrogenation reaction of o-chloronitrobenzene and can enable the o-chloronitrobenzene to perform selective hydrogenation for further generating o-chloroaniline, thereby showing good catalytic hydrogenation performance.
Owner:BEIJING UNIV OF CHEM TECH
Method for preparing o-chloroaniline by catalytic hydrogenation
InactiveCN101774931AEasy to clean regularlyInhibition of dechlorination reactionOrganic compound preparationAmino compound preparationO-nitrochlorobenzeneFixed bed
The invention discloses a method for preparing o-chloroaniline by catalytic hydrogenation. O-nitrochlorobenzene, ethyl acetate solution, a dechlorination inhibitor and hydrogen are subjected to catalytic hydrogenation synthetic reaction under the effect of a catalyst at the temperature of 25-90 DEG C to obtain the o-chloroaniline. For better technical effects, the o-nitrochlorobenzene, ethyl acetate solution, dechlorination inhibitor are continuously delivered to a fixed bed reactor by a metering pump, inlet amount of hydrogen is controlled by a flow meter, and catalytic hydrogenation synthetic reaction is conducted in the fixed bed reactor. The fixed bed reactor which uses Ni alloy as the catalyst and a neutral carrier as a catalyst carrier is used for controlling the inlet amount of raw materials and hydrogen and the catalyst, thus reducing cost and improving efficiency. In the invention, dechlorination reaction can be effectively inhibited, subsequent processing steps of the principal product are simplified, coupling compounds generated in reaction are little, the selectivity of o-chloroaniline is above 99%, and the dechlorinating amount is below 0.3%. The invention has the advantages of mass industrial production and environment protection.
Owner:JIANGSU KANGHENG CHEM
Method for preparing o-chloroaniline by adopting microchannel reaction device
ActiveCN103664642AFully contactedWell mixedOrganic compound preparationAmino compound preparationChlorobenzenePtru catalyst
The invention belongs to the technical field of fine chemical engineering, and relates to a method for preparing o-chloroaniline by adopting liquid-phase catalytic hydrogenation of o-nitrochlorobenzene. A microchannel reaction device is adopted in reaction, and ortho-nitrochlorobenzene, a solvent and a catalyst react with hydrogen by adopting a slurry feeding mode at 50-150 DEGC under the pressure of 0.5-1.2MPa, so as to obtain the o-chloroaniline. The preparation technology of the o-chloroaniline has the advantages that feed is continuous, the reaction condition is flexible to change, mass transfer effect is good, a reactant fully contacts with a catalyst, the side reaction is reduced, and the like.
Owner:CHINA PETROLEUM & CHEM CORP +1
Method for preparing o-chloroaniline
InactiveCN1660774AHigh yieldLow costOrganic compound preparationAmino compound preparationAlcoholSolvent
Owner:ZHEJIANG UNIV OF TECH
Simple and convenient preparation method of key intermediate (2-methyl-4-amino-5-amino methyl pyrimidine) for vitamin B1
The invention relates to a simple and convenient preparation method of a key intermediate (2-methyl-4-amino-5-amino methyl pyrimidine) for the vitamin B1. According to the method, lewis acid is used for catalyzing addition condensation reaction of acetamidine and 3-formyl amino ethyl cyanide and then catalyzing reaction of the condensation product and triethyl orthoformate to introduce a formyl chiral auxiliary, an imdo group and the formyl chiral auxiliary are subjected to ring formation to form 2- methyl-4-formyl amino-5- amino methyl pyrimidine, and finally basic hydrolysis is performed, so that 2-methyl-4-amino-5-amino methyl pyrimidine is obtained. The reaction procedures are carried out sequentially by adopting the one-pot method, the products in each step require no separation and purification, and the operation is simple and convenient. According to the method, highly toxic o-chloroaniline and other phenylamine compounds are not used, so that residue of o-chloroaniline compounds in the vitamin B1 product can be completely avoided. Meanwhile, the production wastewater is less and the yield is high.
Owner:XINFA PHARMA
Method for preparing o-chloroaniline by virtue of solvent-free catalytic hydrogenation
ActiveCN103664641ASolve the problem of hydrogenolysis and dechlorinationReduce corrosionOrganic compound preparationAmino compound preparationPtru catalystSolvent free
The invention belongs to the technical field of fine chemical engineering, and relates to a method for preparing o-chloroaniline by virtue of solvent-free catalytic hydrogenation ortho-nitrochlorobenzene. The method is characterized in that ortho-nitrochlorobenzene is adopted as a raw material to be reacted with hydrogen in the presence of a catalyst at the temperature of 70-120 DEG C and the pressure of 0.5-5.0 MPa so as to obtain o-chloroaniline through the processing after the reaction. By adopting the method, no solvent is added, the defect for adding the solvent can be overcome, the environmental pollution problem and the solvent recycling problem can be avoided, the equipment investment is reduced, the production cost is reduced, the conversion rate of the ortho-nitrochlorobenzene can reach 100 percent, the selectivity of the o-chloroaniline is more than 99.2 percent, and the dechlorinating rate is less than 0.09 percent.
Owner:CHINA PETROLEUM & CHEM CORP +1
Method and device for producing o-chloroaniline without solvent
InactiveCN103387498AReduce labor intensityEasy to operateOrganic compound preparationChemical recyclingSolventPollution
The invention discloses a method and a device for producing o-chloroaniline without a solvent. The method utilizes o-chloronitrobenzene as a raw material, a dechlorination inhibitor as an assistant 1, ammonia water as an assistant 2 and a platinum-carbon catalyst. At a hydrogenation liquid pH of 6-8, under the pressure of 0.5-0.9Mpa, at a temperature of 85-105 DEG C, hydrogen is fed into a reactor and the reaction system undergoes a continuous reaction in the reactor; then the hydrogenation liquid is collected in a hydrogenation liquid tank by a catalyst filter; the catalyst filtered by the catalyst filter is blown back into the reactor by the catalyst filter for recycle; and the hydrogenation liquid subjected to water separation is fed into a rectification system and then is rectified so that a qualified product is obtained. The method and the device have the advantages of simple processes, low production cost, high automation degree, high yield, high output, high reaction selectivity, good product quality, mild reaction conditions, good safety, low labor intensity, no pollution, and easy popularization.
Owner:王一如
Preparation method for catalyst used for preparation of chlorinated arylamines through catalytic hydrogenation
ActiveCN107970967APrevent restoreEvenly distributedPhysical/chemical process catalystsOrganic compound preparationBenzeneAmmonium compounds
The invention relates to a preparation method for a catalyst used for preparation of chlorinated arylamines through catalytic hydrogenation, and specifically to a preparation method for a supported noble metal complex catalyst and an application of the supported noble metal complex catalyst in preparation of the chlorinated arylamines like o-chloroaniline, 3,4-dichloroaniline and 2,5-dichloroaniline through catalytic hydrogenation. The invention provides a preparation method for a carbon-supported catalyst (Pt-N / C or Pd-N / C for short, wherein N represents one or more selected from the group consisting of inorganic ammonium compounds) which is obtained through an action of noble metal and an inorganic ammonium compound; and the catalyst is used for preparation of the chlorinated arylaminesthrough catalytic hydrogenation of chloronitrobenzene. The preparation processes for the catalyst and the chlorinated arylamines have the following main advantages: 1, little difference is generated between preparation processes of the catalyst and ordinary noble metal carbon-supported catalysts, and the preparation processes are simple; 2, continuous addition of an auxiliary agent is not needed in the process of preparation of the chlorinated arylamines through catalytic hydrogenation of chloronitrobenzene by utilizing the catalyst; 3, the catalyst has stable activity and low dechlorination amount in the process of hydrogenation; and 4, no solvent is used in the process of hydrogenation, and production capacity is improved.
Owner:JIANGSU RUIXIANG CHEM +1
Treatment for waste water of benzidine production by two-section adsorbing method an d resource recovery method
InactiveCN101012073ACombustible gas purificationWater/sewage treatment by neutralisationHigh densityDesorption
The invention discloses a processing method of benzidine manufacturing wastewater and resource recycling method, which comprises the following steps: adjusting pH value of benzidine manufacturing wastewater to 4-7; adsorbing through first composite functional resin; recycling o-chloroaniline; adjusting adsorbed water to 7-10; adsorbing through second composite functional resin; reducing COD below 500mg / L and o-chloroaniline below 5mg / L; adsorbing first composite functional resin through HCl solution completely; obtaining high-density desorption liquid; neutralizing through NaOH solution; separating; recycling o-chloroaniline with density over 98%; regenerating the second composite functional resin through the same method; allocating the first resin through high-density desorption liquid; reducing the discharge quantity of organic pollution.
Owner:NANJING UNIV +1
Composite flame retardant modified polyurethane composite material
The invention discloses a composite flame retardant modified polyurethane composite material, the raw materials of which include: polyether polyol, 4,4'-diphenylmethane diisocyanate, toluene diisocyanate, epoxy resin, acrylic resin, pentaerythritol tri Allyl Ether, Anilinomethyltriethoxysilane, Trimethylolpropane, Dibromoneopentyl Glycol, Dibutyltin Dioctoate, Dimethylthiotoluenediamine, 4,4'-Methylenebis-Ortho Chloroaniline, attapulgite, montmorillonite, hollow glass microspheres, white carbon black, expanded vermiculite, multi-walled carbon nanotubes, epoxidized natural rubber, composite flame retardant; composite flame retardant consists of expandable graphite, A mixture of zinc stannate and P‑N‑Si flame retardant. The composite flame retardant modified polyurethane composite material proposed by the invention has high strength and excellent flame retardancy, and can meet the use requirements in various fields.
Owner:CHUZHOU GLOBAL POLYURETHANE TECH CO LTD
Non-polluted method for producing o-chloroaniline with ferrous powder as reducer
InactiveCN101376634AReduce energy consumptionReduce productionOrganic compound preparationAmino compound preparationO-nitrochlorobenzeneIron powder
The invention relates to an environment friendly method for reduction producing o-chloroaniline with iron powder. The method comprises adopting o-nitrochlorobenzene as the raw material and the iron powder as a reducing agent; separating o-chloroaniline, water andiron mud by vacuum distilling to obtain a coarse o-chloroaniline product; and refining by rectifying to obtain o-chloroaniline. The method solves the defects in the prior art on heavy pollution and high energy consumption, and implements the clear production of o-chloroaniline.
Owner:淮安嘉诚高新化工股份有限公司
Organic electroluminescence material with heterocyclic ring structure as well as preparation method and application thereof
InactiveCN109020979ANot easy to crystallizeNot easy to gatherOrganic chemistrySolid-state devicesHydrogenBoric acid
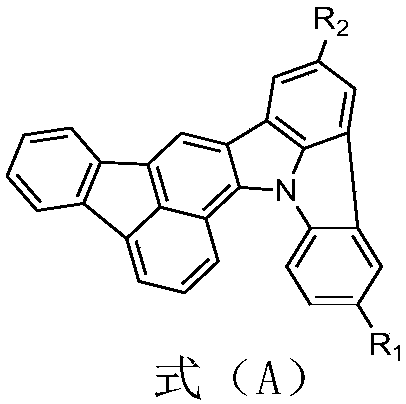
The invention relates to an organic electroluminescence material with a heterocyclic ring structure as well as a preparation method and application thereof. The structure formula of the organic electroluminescence material with the heterocyclic ring structure is shown in the description, wherein the R1 and the R2 are any one kind of materials from hydrogen, aromatic heterocyclic groups with the carbon atom number being 5 to 60 and polycyclic aromatic group conjugated structure groups with the carbon atom number being 5 to 60; the R1 and the R2 are identical or different. The organic electroluminescence material with the heterocyclic ring structure is obtained by using 3-bromofluoranthene, o-chloroaniline, o-chlorobromobenzene, halogenated reagents and boric acid compounds as raw materialsthrough coupling reaction under the effects of catalysts and basic substances. The material is applied to a luminescence layer in an organic electroluminescence device; crystallization and gathering cannot easily occur between material molecules; good film stability is realized. The organic electroluminescence material device prepared by the material has lower driving voltage and high current efficiency; good photoelectric performance is realized.
Owner:VALIANT CO LTD
Chemical mechanical polishing pads for improved removal rate and planarization
ActiveUS20180345448A1Favorable shear storage modulusHigh (Lapping machinesLapping toolsPrepolymerToluene diisocyanate
The present invention provides a chemical mechanical (CMP) polishing pad for polishing three dimensional semiconductor or memory substrates comprising a polishing layer of a polyurethane reaction product of a thermosetting reaction mixture of a curative of 4,4′-methylenebis(3-chloro-2,6-diethylaniline) (MCDEA) or mixtures of MCDEA and 4,4′-methylene-bis-o-(2-chloroaniline) (MbOCA), and a polyisocyanate prepolymer formed from one or two aromatic diisocyanates, such as toluene diisocyanate (TDI), or a mixture of an aromatic diisocyanate and an alicyclic diisocyanate, and a polyol of polytetramethylene ether glycol (PTMEG), polypropylene glycol (PPG), or a polyol blend of PTMEG and PPG and having an unreacted isocyanate (NCO) concentration of from 8.6 to 11 wt. %. The polyurethane in the polishing layer has a Shore D hardness according to ASTM D2240-15 (2015) of from 60 to 90, a shear storage modulus (G′) at 65° C. of from 125 to 500 MPa, and a damping component (G″ / G′ measured by shear dynamic mechanical analysis (DMA), ASTM D5279-08 (2008)) at 50° C. of from 0.06 to 0.13.
Owner:ROHM & HAAS ELECTRONICS MATERIALS CMP HLDG INC +1
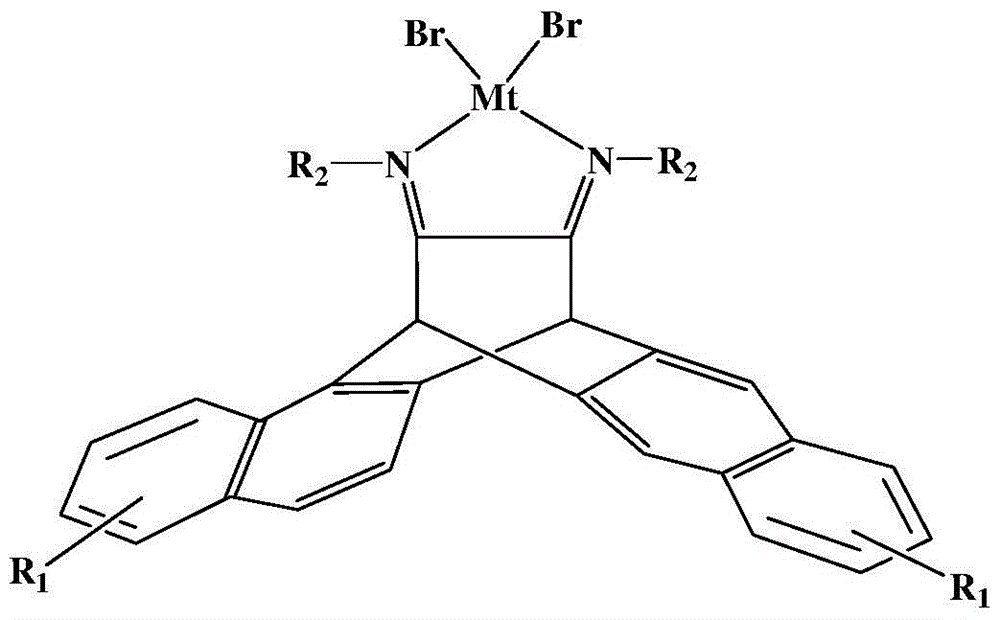
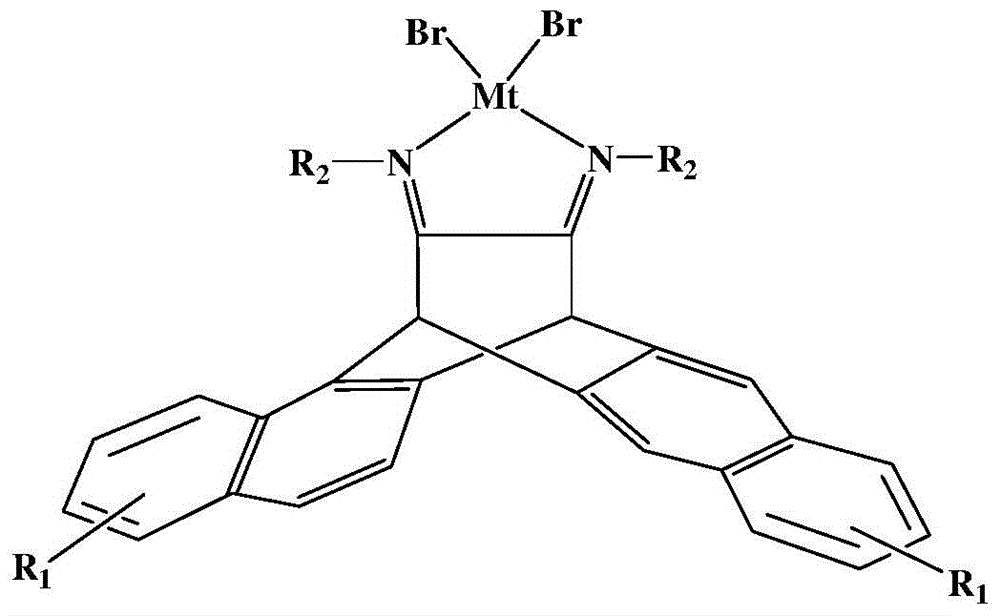
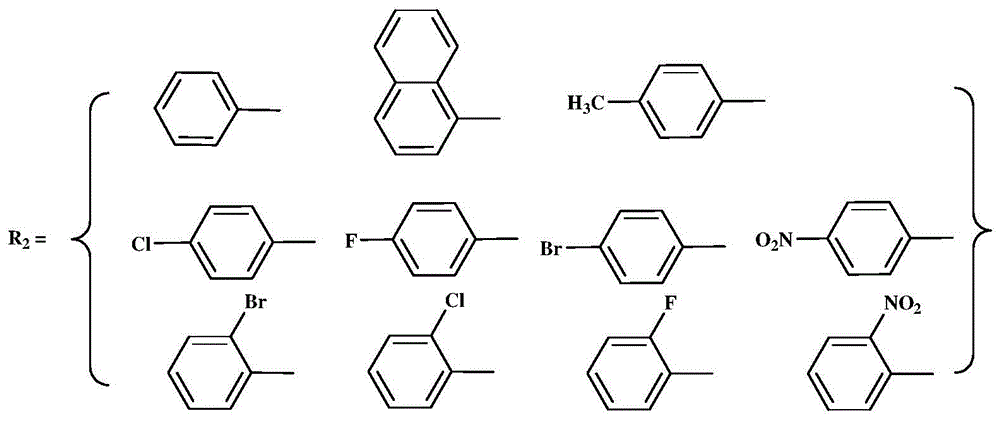
N,N-single ligand metal catalyst with three-dimensional structure and preparation method thereof
InactiveCN105936659AEasy to processGood light transmissionNickel organic compoundsDimethylaniline N-oxideP-Nitroaniline
The invention provides an N,N-single ligand metal catalyst with three-dimensional structure and a preparation method thereof. The catalyst has a structural general formula as below. Mt represents nickel, palladium, cobalt, iron or copper metal atom; R1 represents bromine, chlorine, iodine, phenyl group, propyl group, butyl group, phenyl group, naphthyl group, methoxy group or ethoxy group; when R2 is aromatic amine, it represents aniline, naphthylamine, p-methylaniline, p-chloroaniline, p-fluoroaniline, p-fluoroaniline, p-bromoaniline, p-nitroaniline, o-bromoaniline, o-chloroaniline, o-nitraniline, 2,6-dichloroaniline, 2,6-difluoroaniline, 2,6-dibromoaniline, 2,6-dinitroaniline, 2,6-dimethylaniline, 2,6-diisopropyl aniline, 2,4-difluoroaniline, 2,4-dibromoaniline, 2,4-dichloroaniline, 2,4,6-trichloroaniline, 2,4,6-tribromo aniline, 2,4,6-trifluoroaniline, 2,4,6-trinitroaniline, 6-hydroxynaphthylamine, 1-aminoindan, fluoreneamine or 4-nitro naphthylamine; and when R2 is aliphatic amine, it represents ethylamine, propylamine, butylamine, heptylamine, isopropylamine, tert-butylamine or cyclohexylamine. The preparation method is simple, less in by-products, and environmentally friendly.
Owner:YICHUN UNIVERSITY +1
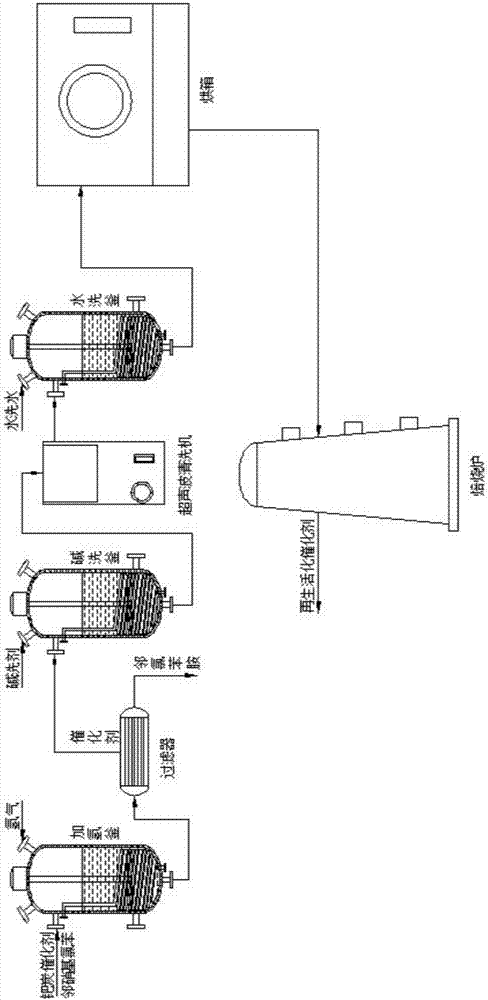
Method for regenerating catalyst for synthesizing o-chloroaniline
InactiveCN107570215AImprove regeneration efficiencyImprove recycling ratesOrganic-compounds/hydrides/coordination-complexes catalystsMetal/metal-oxides/metal-hydroxide catalystsEconomic benefitsMegasonic cleaning
The invention discloses a method for regenerating a catalyst for synthesizing o-chloroaniline. The method for regenerating the catalys specifically includes the following steps of: filtering a palladium-carbon catalyst out in a filter which is provided with a metal sintered tube and arranged in a final-stage kettle of four-stage series hydrogenation kettles, introducing the palladium-carbon catalyst into an alkaline washing kettle, conducting alkaline washing on the palladium-carbon catalyst with a hot alkaline solution, introducing the alkaline-washed palladium-carbon catalyst into an ultrasonic cleaner to perform ultrasonic cleaning, introducing the ultrasonic-cleaned palladium-carbon catalyst into a water washing kettle for water washing, putting the water-washed palladium-carbon catalyst into an oven for drying and water removal, and then raising the temperature and calcining the dried palladium-carbon catalyst to obtain the dried and regenerated palladium-carbon catalyst. According to the method for regenerating the catalyst for synthesizing the o-chloroaniline, the useful palladium-carbon catalyst can be effectively recycled, the content of o-phenylenediamine obtained throughthe catalysis of the palladium-carbon catalyst is 98% or above, the synthesis cost is saved, and the economic benefit is improved; besides, the method and the process design of the regeneration of the catalyst used for synthesizing the o-chloroaniline are optimized, and requirements of the industrial design are met.
Owner:ANHUI DONGZHI GUANGXIN AGROCHEMICAL CO LTD
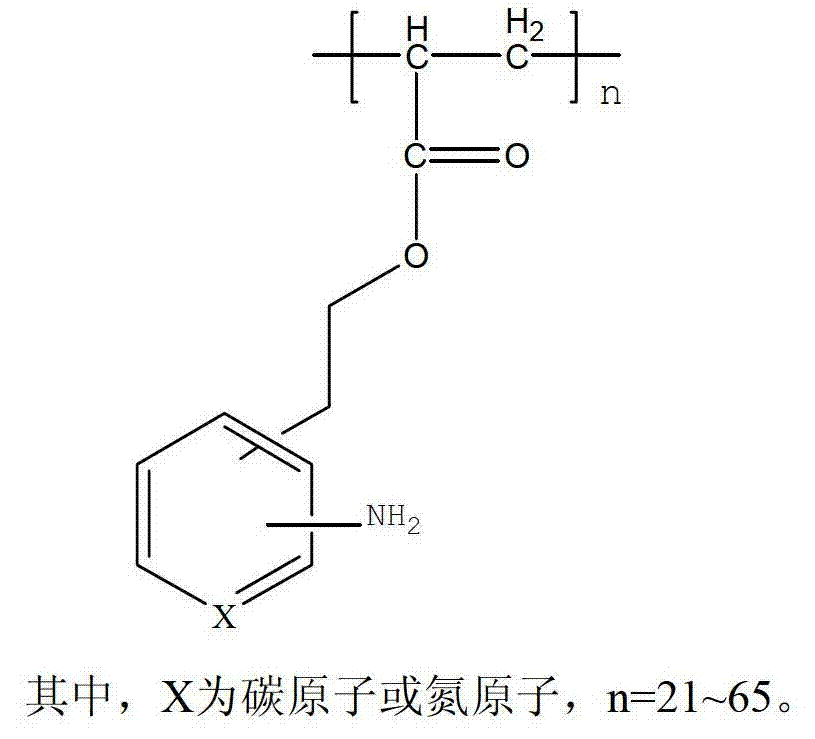
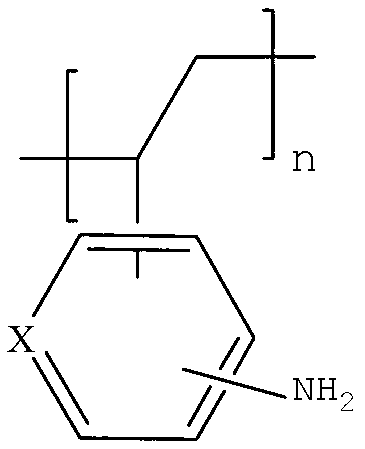
Amino arene structure unit-containing polyacrylate functionalized polymer and preparation method thereof
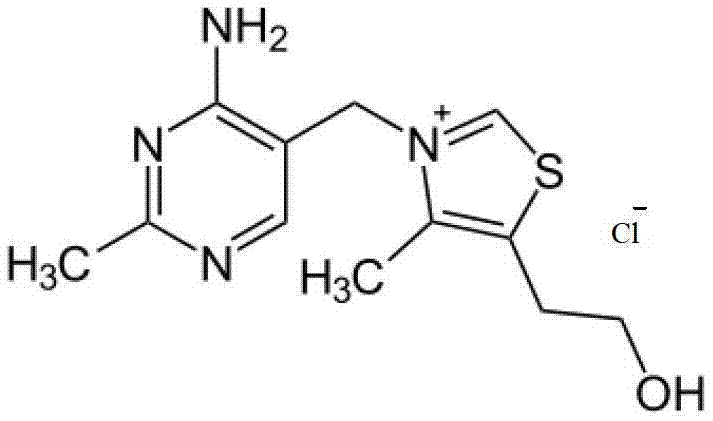
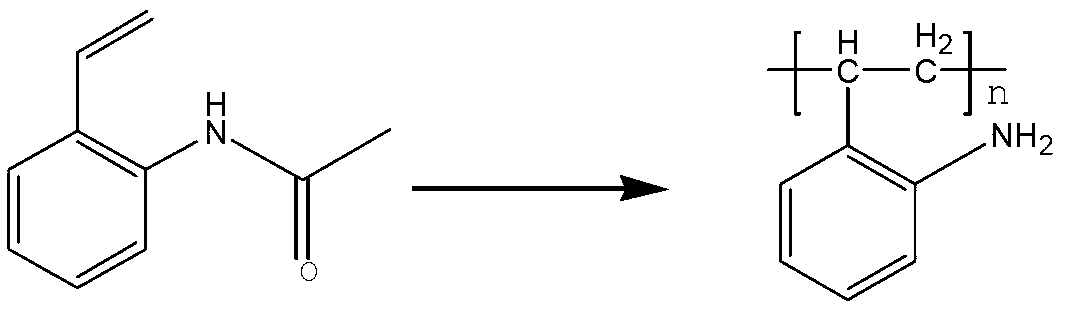
The invention relates to an amino arene structure unit-containing polyacrylate functionalized polymer and a preparation method thereof. The preparation method comprises the steps of: by utilizing a method of initiating solution polymerization through free radicals, initiating an amino arene structure unit-containing polyacrylate monomer to be in free radical polymerization in an alcohol-water solvent, performing molecular weight regulation to the polymer by lauryl mercaptan, separating according to different solubilities of polymers with different molecular weights in the alcohol-water system to obtain the amino arene structure unit-containing polyacrylate functionalized polymer with the molecular weight range of 4,000-12,000. The functionalized polymer can be used for entirely replacing o-chloroaniline for preparing vitamin B1, can be recycled after being filtered, the preparation method is simple and convenient to operate, and high in yield, thereby being an industrialized green and environment-friendly production method with the raw material cost advantage.
Owner:XINFA PHARMA
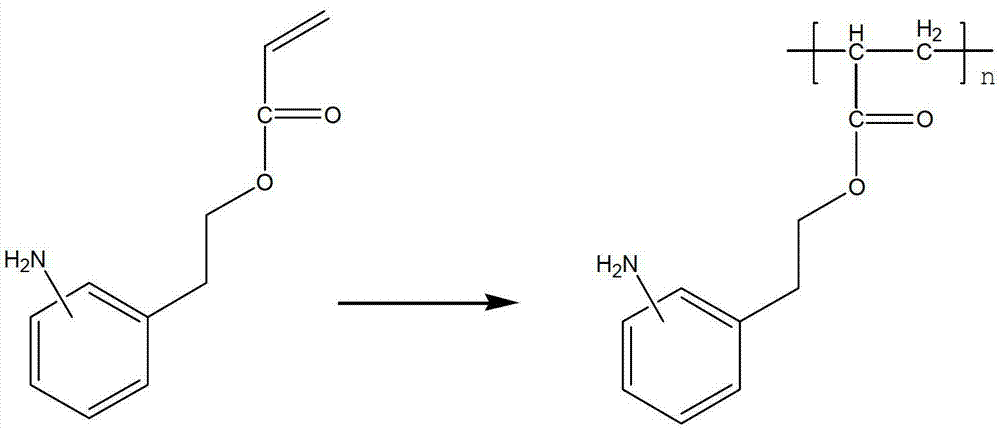
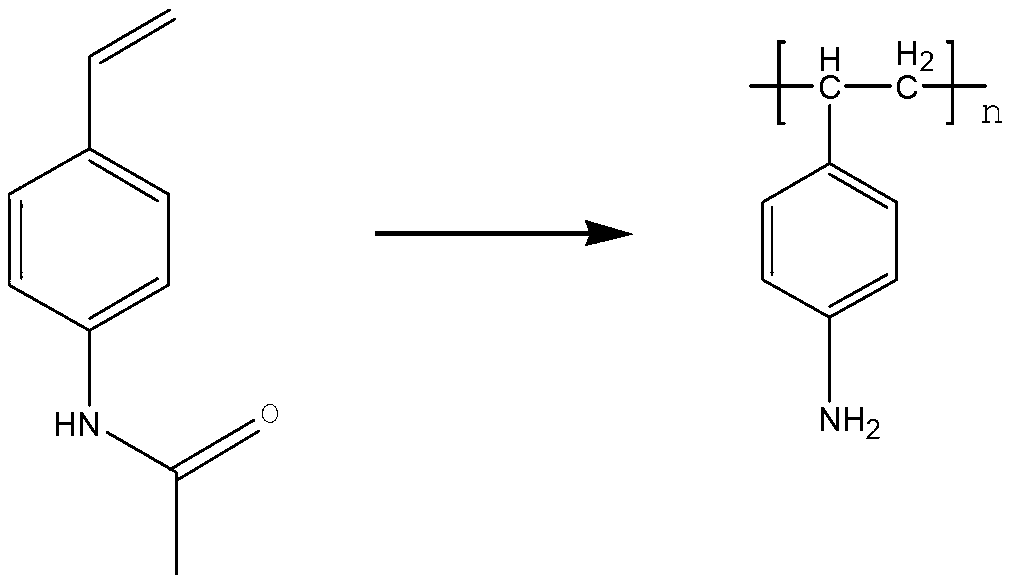
Functional polymer containing amino aryl ethylene and preparation method of functional polymer
The invention relates to a functional polymer containing amino aryl ethylene and a preparation method of the functional polymer. The preparation method comprises the following steps of: initiating free radical polymerization of a monomer acetamino aryl ethylene in an alcohol-water solvent by using a method for initiating solution polymerization by utilizing free radicals; then, adjusting the molecular weight of the polymer by using lauryl mercaptan; and separating in an alcohol-water system to obtain the functional polymer containing amino aryl ethylene, wherein the molecular weight of the functional polymer is within a certain range. The polymer can be dissolved or most of the polymer can be dissolved in the hot alcohol-water system; and the polymer can be used for substituting for highly-cancerogenic o-chloroaniline or other micromolecular aniline derivatives to prepare a key intermediate 2-methyl-4-amino-5-aminomethyl pyrimidine of vitamin B1. The functional polymer containing amino aryl ethylene can be recycled after being filtered; the polymer can be used for completely substituting for o-chloroaniline to prepare vitamin B1; and the preparation method is simple and convenient to operate and high in yield.
Owner:XINFA PHARMA
O-chloroaniline preparation method
InactiveCN110655470AHigh purityInhibition of mass transferAmino compound purification/separationOrganic compound preparationPtru catalystFluid phase
The invention provides ano-chloroaniline preparation method, wherein the raw materials comprise: o-nitrochlorobenzene, a catalyst, dicyandiamide, a solvent, nitrogen and hydrogen. According to the invention, Ru / C is used as the catalyst, and has characteristics of simple components, stable activity and high selectivity, and dicyandiamide is used as the dechlorination inhibitor, so that the dechlorination side reaction is effectively inhibited, the conversion rate is high, and the selectivity is high; by improving the structure of the rectifying tower, the vapor-liquid mass transfer performancein heat transfer and mass transfer is improved, and the contact area between the gas phase and the liquid phases is increased by utilizing the auxiliary member; the turbulent flow degree is effectively increased through the inflow weir having the step structure and the turbulent flowblock, so that the mass transfer and heat exchange efficiency and the mass transfer and heat exchangerate between the gas phase and the liquid phases are improved; and the treatment and circulation capacities of the liquid phase are improved through the circulation tank, and the gas-liquid phase contact time is favorably prolonged by using the baffle arrangement of the downcomer and the inner diameter change, so that the yield and the purity of o-chloroaniline are improved.
Owner:滨海县星光化工有限公司
Aurora kinase A inhibitor and preparation and application thereof
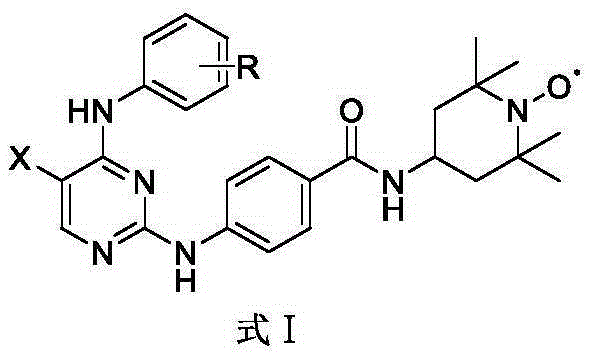
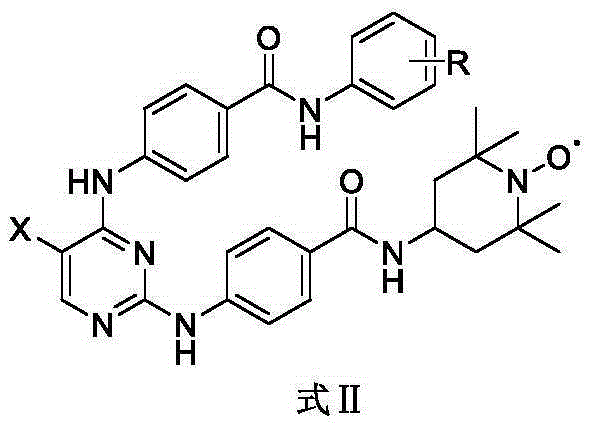
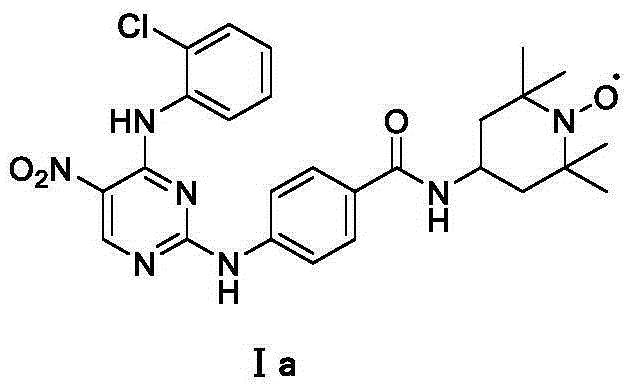
InactiveCN106674197AGood inhibitory effectEnhanced inhibitory effectOrganic chemistryAntineoplastic agentsNitrogen oxides2,2,6,6-Tetramethylpiperidine
The invention discloses a novel stable nitroxide radical-marked aurora kinase A inhibitor and a preparation method and application thereof. The aurora kinase A inhibitor is N-(5-fluoro-4-o-chloroaniline-pyrimidin-2)-aminobenzoic acid 4-amino-2, 2, 6, 6-tetramethylpiperidyl oxynitride amide, or N-{5-fluoro-4-amino-(benzoyl o-chloroaniline)-pyrimidin-2}-aminobenzoic acid 4-amino-2, 2, 6, 6-tetramethylpiperidyl oxynitride amide. According to the preparation method, a target compound is prepared by performing a substitution reaction on 2, 4-dichloro-5-substituted-pyrimidine and aniline or p-aminobenzanilide and then 4-amino-2, 2, 6, 6-tetramethylpiperidyl nitrogen oxide. The aurora kinase A inhibitor is applied to preparation of anticancer medicines.
Owner:LANZHOU UNIVERSITY
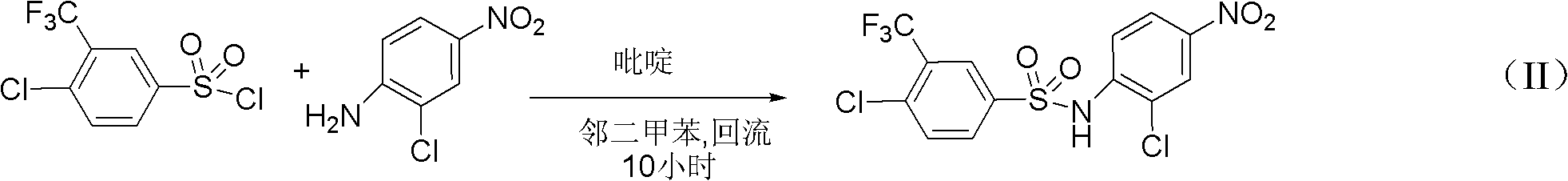
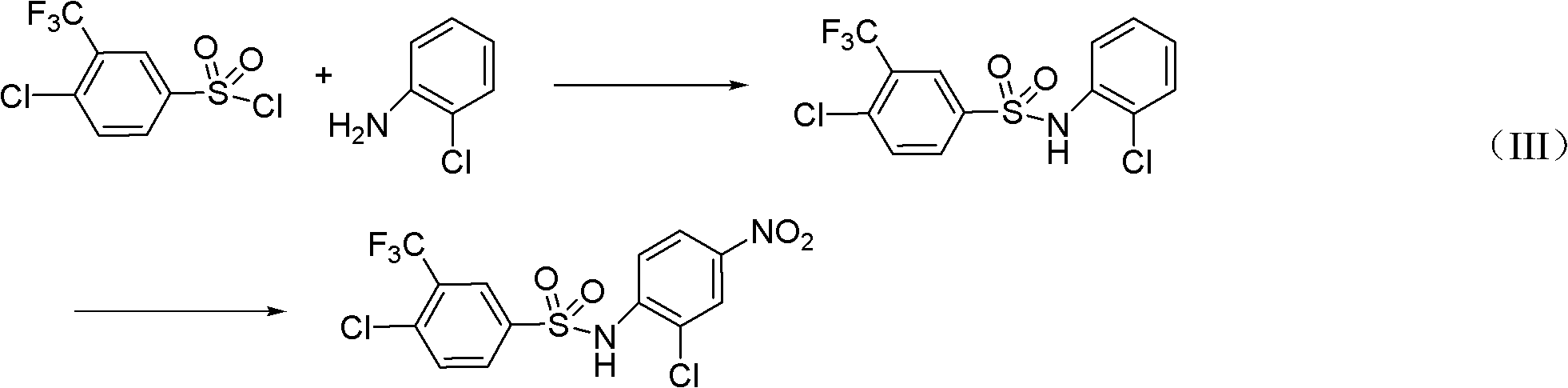
Preparation method for N-(2- chlorine-4-phenyl)-4- chlorine-3-trifluoromethyl benzene sulfonamide
InactiveCN102491924AHigh reaction yieldReduce production risksSulfonic acid amide preparationSulfonyl chlorideNitration
The invention discloses a preparation method for N-(2- chlorine-4-phenyl)-4- chlorine-3-trifluoromethyl benzene sulphonamide. The preparation method is as follows: performing amino sulfonylation reaction to 4 - chlorine-3-trifluoromethyl benzene sulfonyl chloride and o-chloroaniline in the toluene solution in the presence of catalyst to acquire N-(2- chlorine-4-phenyl)-4- chlorine-3-trifluoromethyl benzene sulphonamide; and then, performing nitratlon reaction in the presence of nitric acid / sulphuric acid to acquire flusulfamide with the product purity more than 99%. The preparation method improves the reaction yield, reduces the production danger, and improves the production process on the basis of improving the reaction yield.
Owner:天津均凯农业科技有限公司
Method for preparing vitamin B1 intermediate (pyrimidine)
The invention discloses a method for preparing a vitamin B1 intermediate (pyrimidine). The method comprises the following steps: directly carrying out water vapor distillation to recover o-chloroaniline, hydrolyzing, adding methanol, and properly controlling the aminopyrimidine concentration and the methanol concentration to obtain the intermediate thiothiamine used in a next process. The method has the advantages of simple process operation, high combined yield, environmental protection and energy saving.
Owner:江苏兄弟维生素有限公司
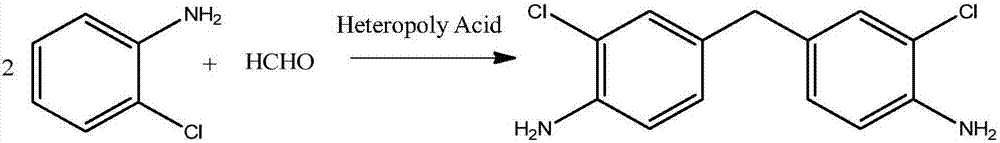
Method of synthesizing 3,3'-dichloro-4,4'-diaminodiphenylmethane by using heteropoly acid
InactiveCN107417544AReduce pollutionEasy to operateOrganic compound preparationAmino compound preparationHeteropoly acidRoom temperature
The invention discloses a method of synthesizing 3,3'-dichloro-4,4'-diaminodiphenylmethane by using heteropoly acid. The method comprises the following steps: (1) adding o-chloroaniline and a formaldehyde solution into a reaction vessel in a room temperature; (2) adding a heteropoly acid catalyst into the reaction vessel; (3) under a magnetic stirring condition, performing heating to ensure the temperature in a range of 50-160 DEG C and performing a reaction for 0.5-8 hours; (4) after the reaction is completed, performing cooling to the room temperature, performing pumping filtration, removing the filtrate, and collecting the filter cake; and (5) performing recrystallization on the obtained filter cake by using an organic mixed solvent, and performing filtering and drying to obtain the solid heteropoly acid synthesized 3,3'-dichloro-4,4'-diaminodiphenylmethane. The method provided by the invention is mild in condition, simple in operation, high in yield and good in product quality.
Owner:NINGBO INST OF TECH ZHEJIANG UNIV ZHEJIANG
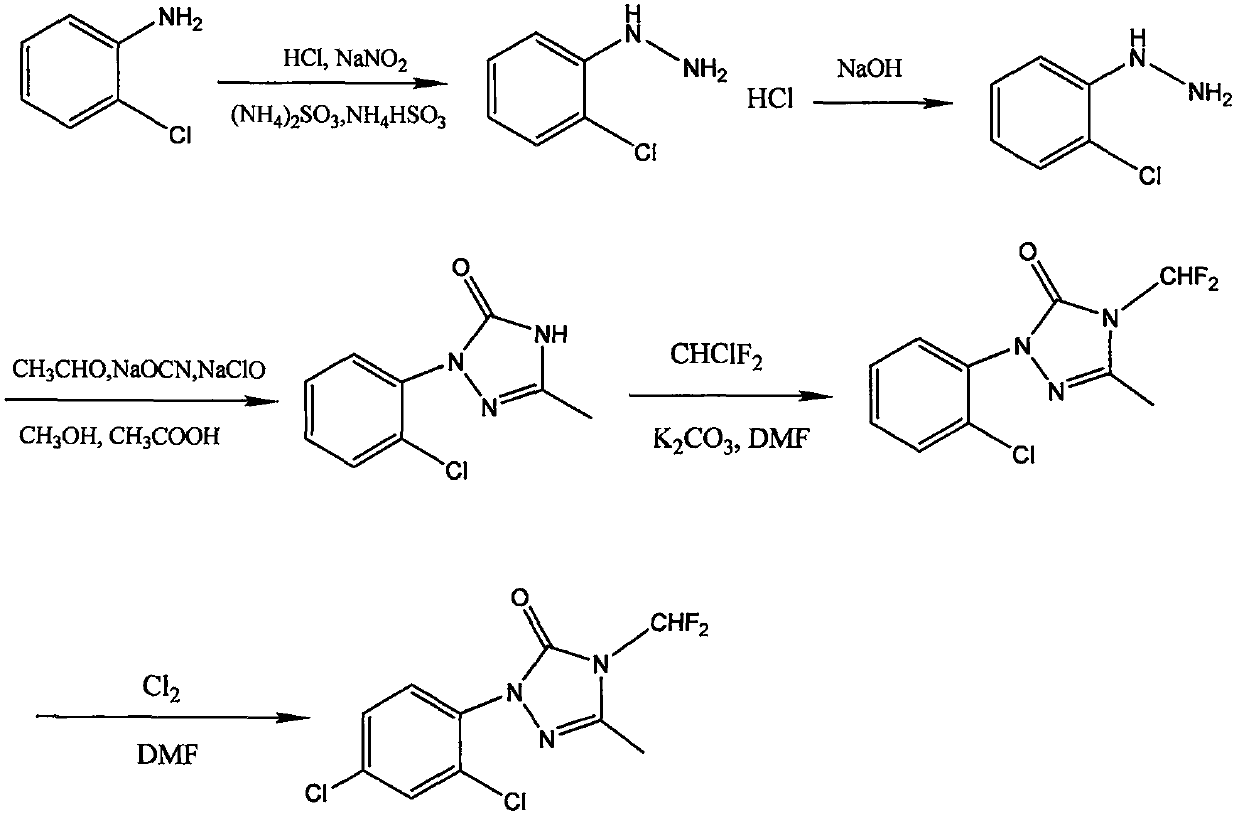
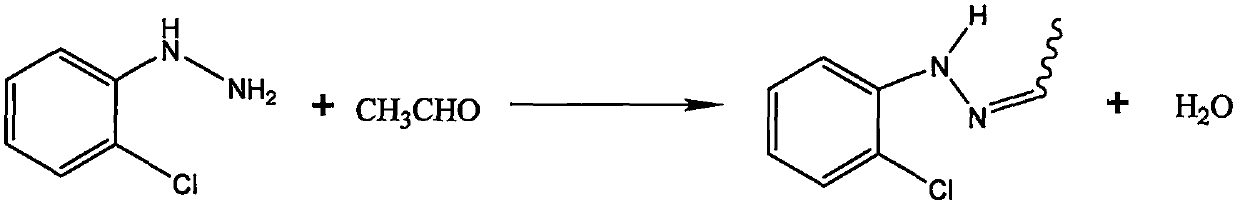
Method for synthesizing 1-(2,4-dichlorophenyl)-3-methyl-4-difluoromethyl-1,2,4-triazole-5-ketone
The invention discloses a method for synthesizing 1-(2,4-dichlorophenyl)-3-methyl-4-difluoromethyl-1,2,4-triazole-5-ketone. The method comprises the following steps: with o-chloroaniline as a raw material, carrying out diazotization, reduction and salification so as to synthesize o-chlorophenylhydrazine hydrochloride, carrying out alkali neutralization so as to prepare free o-chlorophenylhydrazine, subjecting o-chlorophenylhydrazine, acetaldehyde, sodium cyanate and sodium hypochlorite to condensation, cyclization and oxidation reactions so as to generate 1-o-chlorophenyl-3-methyl-1H-1,2,4-triazole-5-ketone, subjecting 1-o-chlorophenyl-3-methyl-1H-1,2,4-triazole-5-ketone to salification, potassium fluorination and chlorination reactions so as to obtain 1-(2,4-dichlorophenyl)-3-methyl-4-difluoromethyl-1,2,4-triazole-5-ketone (a sulfentrazone intermediate). The synthetic method provided by the invention uses o-chloroaniline as the raw material, can synthesize an intermediate with small steric hindrance and few impurities, improves the yield, has the advantages of easily-available and cheap raw materials, mild reaction, simple and convenient operation and high safety, and facilitatesindustrial production.
Owner:连云港世杰农化有限公司
Preparation of a nitrogen-doped carbon-coated core-shell nickel-iron alloy nanocatalyst and its application in the hydrogenation of o-chloronitrobenzene
ActiveCN106732733BMaterial nanotechnologyPhysical/chemical process catalystsNitro compoundNano catalyst
The invention provides a preparation method of a nitrogen-doped carbon-coated core-shell structure nickel-iron alloy nanocatalyst in the field of catalyst technology and its application in catalyzing the hydrogenation reaction of o-chloronitrobenzene. The method first synthesizes a nickel-iron layered double metal hydroxide precursor with small particle size and high surface energy through a nucleation and crystallization isolation method, and then uniformly mixes it with a melamine and dicyandiamide mixed carbon material precursor. , and finally a new type of nitrogen-doped carbon-coated core-shell structure nickel-iron alloy nanocatalyst was prepared through high-temperature self-reduction. It is efficiently used in the catalytic hydrogenation of halogenated nitro compounds to produce halogenated anilines. The conversion rate of o-chloronitrobenzene and the selectivity of o-chloroaniline can reach 95-100% and 98-100% respectively. . This new nitrogen-doped carbon-coated core-shell structure nickel-iron alloy nanocatalyst has a novel and unique structure, a green and energy-saving process, and a stable catalyst structure, and has broad application prospects.
Owner:BEIJING UNIV OF CHEM TECH
Synthesis method of sulfentrazone intermediate
ActiveCN113402472AReduce manufacturing costAvoid supply constraintsOrganic chemistryP-chloroanilineChlorobenzene
The present invention provides a synthesis method of asulfentrazone intermediate. The methodcomprises: S1) carrying out a nitration reaction on chlorobenzene in a nitration reagent to obtain a mixture of o-chloronitrobenzene and p-chloronitrobenzene, wherein the product does not need to be separated; s2) performing catalytic hydrogenation reaction on the mixture of o-chloronitrobenzene and p-chloronitrobenzene to obtain a mixture of o-chloroaniline and p-chloroaniline, wherein the product does not need to be separated; s3) making the mixture of o-chloroaniline and p-chloroaniline subjected to a diazotization reaction to obtain a mixture of o-chlorophenylhydrazine and p-chlorophenylhydrazine, wherein the product does not need to be separated; s4) performing condensation reaction on the mixture of the o-chlorophenylhydrazine and the p-chlorophenylhydrazine and aldehyde to obtain triazole ring mixtures as shown in a formula I-a and a formula I-b; and S5) carrying out chlorination reaction on the triazole ring mixtures to obtain the sulfentrazone intermediate shown in the formula I. According to the method, 2, 4-dichloroaniline is not used as a raw material, so that the production cost of sulfentrazone is reduced, and the limitation of raw material supply is avoided.
Owner:SHANDONG WEIFANG RAINBOW CHEM
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com