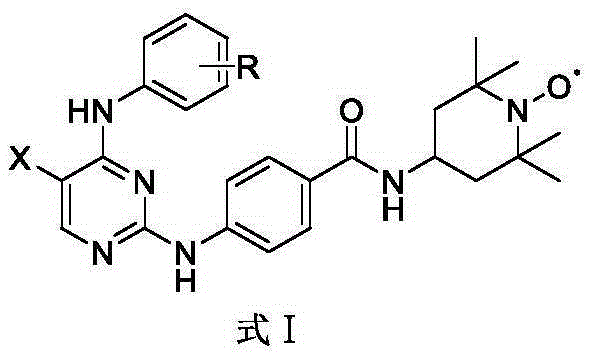
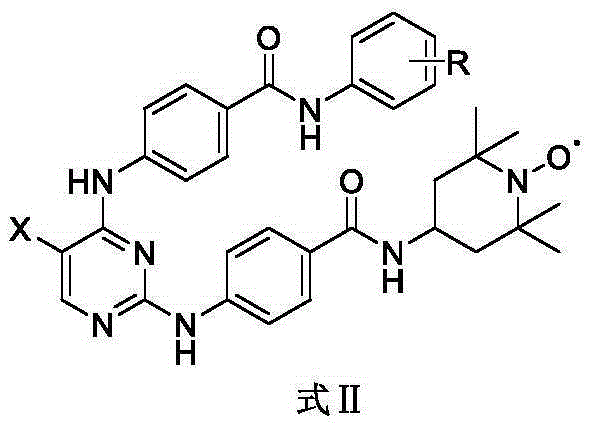
Aurora kinase A inhibitor and preparation and application thereof
A Aurora kinase and inhibitor technology, applied in the field of 2,4-diaminopyrimidine Aurora kinase A inhibitors, can solve the problems of loss, low anti-tumor activity, and poor solubility membrane penetration ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
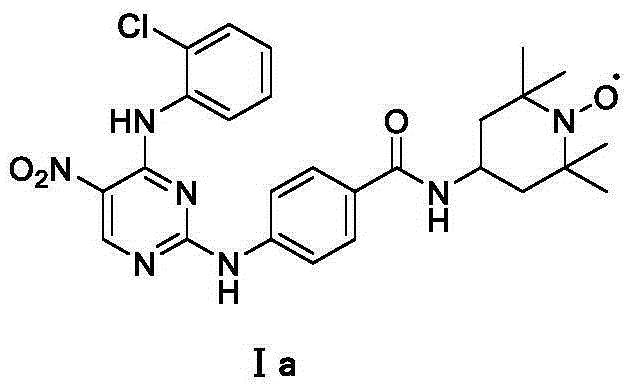
[0023] Embodiment 1: Preparation of 2-chloro-4-o-chloroaniline-5-nitropyrimidine
[0024] Dissolve 2,4-dichloro-5-nitropyrimidine (205mg, 1.05mmol) in dry dichloromethane, add o-chloroaniline (125mg, 1.0mmol) at room temperature and continue the reaction for 8h, evaporate the solvent after the reaction , The residue was directly chromatographed to obtain the product 2-chloro-4-o-chloroaniline-5-nitropyrimidine. Yield: 89%; Yellow solid; 1 H-NMR (600MHz, CDCl 3 )δ10.68 (s, 1H), 9.22 (s, 1H), 8.34-8.32 (m, 1H), 7.51-7.21 (m, 3H).
[0025]Use m-chloroaniline, or p-chloroaniline, or o-hydroxyaniline, or m-hydroxyaniline, or p-hydroxyaniline to replace o-chloroaniline respectively to prepare 2-chloro-4-m-chloroaniline-5-nitropyrimidine, or 2- Chloro-4-p-chloroaniline-5-nitropyrimidine, or 2-chloro-4-o-hydroxyaniline-5-nitropyrimidine, or 2-chloro-4-m-hydroxyaniline-5-nitropyrimidine, or 2 -Chloro-4-p-hydroxyaniline-5-nitropyrimidine.
[0026] Reaction with 2,4-dichloro-5-fluor...
Embodiment 2
[0027] Example 2: Preparation of p-aminobenzoic acid 4-amino-2,2,6,6-tetramethylpiperidine nitrogen oxide amide
[0028] Dissolve 1.8g of Boc-protected p-aminobenzoic acid in dry dichloromethane, and add 1.8g of EDC, 1.22g of HOBt and 1.53g of compound TMPO in sequence. Stir at room temperature until the reaction is complete. The organic phase was washed with water, and dried over anhydrous magnesium sulfate; the solvent was evaporated and column chromatography was performed to obtain 2.52 g of pink solid, with a yield of 90%. MS(ESI)392.15[M+2H] + .
[0029] Weighed 2.5 g of the product obtained from the above reaction and dissolved it in 15 ml of dichloromethane, added 6 ml of trifluoroacetic acid dropwise, stirred and reacted at room temperature for 0.5 h, evaporated the solvent under reduced pressure, and obtained 1.74 g of white solid by column chromatography of the obtained solid, yield 94%. MS(ESI)292.15[M+2H] + .
Embodiment 3
[0030] Example 3: Preparation of N-(5-nitro-4-o-chloroaniline-pyrimidine-2)-aminobenzoic acid 4-amino-2,2,6,6-tetramethylpiperidine nitrogen oxide amide
[0031] 2-Chloro-4-o-chloroaniline-5-nitropyrimidine (280 mg, 1 mmol) and p-aminobenzoyl 4-amino-2,2,6,6-tetramethylpiperidine nitrogen oxide (430 mg, 1.5mmol) was dissolved in ethanol, then 2ml of hydrochloric acid in dioxane was added, and heated to reflux. After the reaction was almost complete, the reaction was stopped, the solvent was evaporated, and a yellow solid was obtained by direct column chromatography, yield: 62%.
[0032]
[0033] m.p.:208-210℃;HPLC:MeOH:H 2 O(0.1%TFA)=65:35, 0.8ml / min, t=21.23min, 95.0%; IR(KBr,cm -1 ) 3362, 3015, 1629, 1536, 1506, 1422, 1332, 1195, 1025, 836, 726; ESR (DMSO): g = 2.006, An (G) = 15.96, △ H (G) = 2.95; HRMS (ESI) 540.2111 for[M+2H] + (calcd 540.2121 for C 26 h 31 N 7 o 4 Cl).
[0034] In the cell proliferation inhibition experiment described later, the sample number...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com