Preparation of a nitrogen-doped carbon-coated core-shell nickel-iron alloy nanocatalyst and its application in the hydrogenation of o-chloronitrobenzene
A nano-catalyst, o-chloronitrobenzene technology, applied in the preparation of organic compounds, preparation of amino compounds, physical/chemical process catalysts, etc., can solve the problems of easy agglomeration and deactivation, expensive noble metal catalysts, poor stability, etc., to achieve High catalytic activity, enhanced electron transport properties and chemical reactivity, high stability effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] 4.04gFe(NO 3 ) 3 9H 2 O, 8.724gNi(NO 3 ) 2 ·6H 2 O was dissolved in 100mL deionized water, ultrasonicated for 5min, mixed thoroughly, and recorded as solution A.
[0026] 2.56 g NaOH and 125 μL of 30% H 2 o 2Dissolved in 100mL deionized water, ultrasonicated for 5min, mixed thoroughly, and recorded as solution B.
[0027] Slowly mix the two solutions at room temperature and add them to the colloid mill at a controlled speed of 3000rpm. After stirring vigorously for 2 minutes, transfer them to a polytetrafluoroethylene liner. After airtight, statically crystallize at room temperature for 24 hours. After the reaction, centrifuge and wash To neutrality, freeze-dry to obtain the NiFe-LDH precursor of the nanocatalyst.
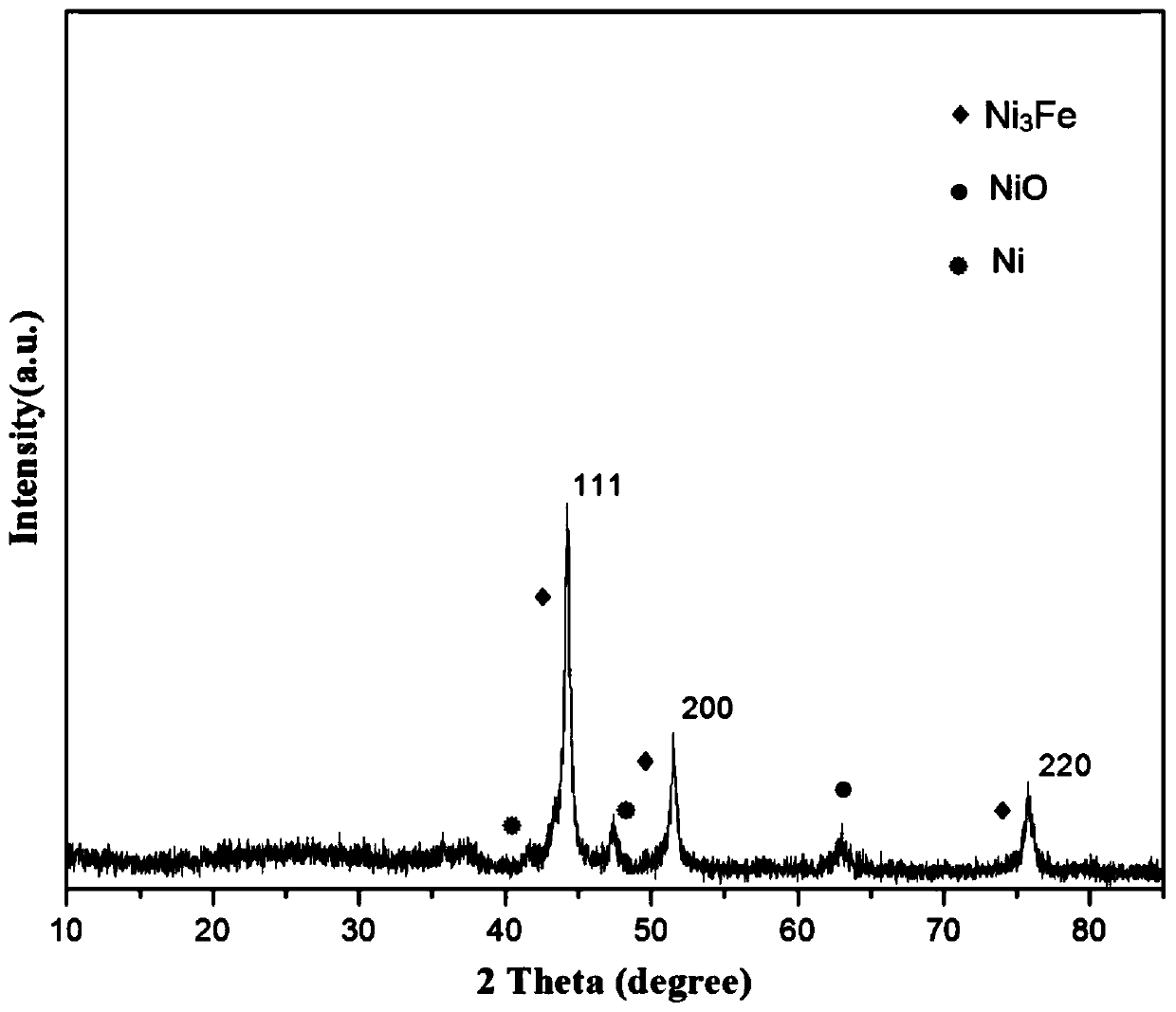
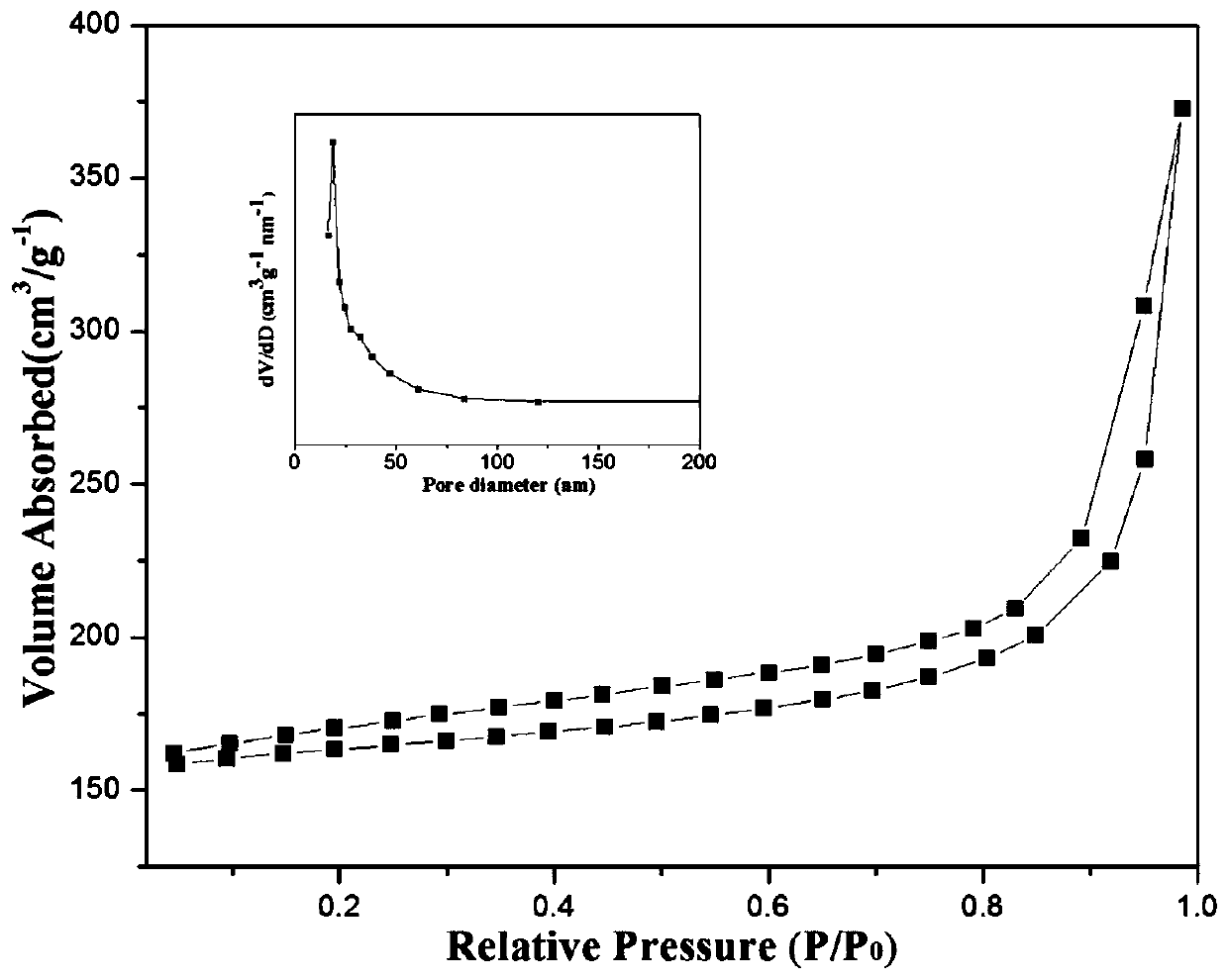
[0028] Take 0.5g of NiFe-LDH, 0.19g of dicyandiamide and 1.31g of melamine and grind them thoroughly in a mortar until they are evenly mixed. -1 Heating up to 500°C and keeping it warm for 6 hours, after grinding, a nano-catalyst with a core-shell s...
Embodiment 2
[0032] 4.04gFe(NO 3 ) 3 9H 2 O, 5.816gNi(NO 3 ) 2 ·6H 2 O was dissolved in 100mL deionized water, ultrasonicated for 5min, mixed thoroughly, and recorded as solution A.
[0033] 2.56 g NaOH and 125 μL of 30% H 2 o 2 Dissolve in 100mL deionized water, sonicate for 5min, and mix well. Denoted as solution B.
[0034] The two solutions were slowly mixed at room temperature and added to the colloid mill at a controlled speed of 3000 rpm. After being vigorously stirred for 2 minutes, they were transferred to a polytetrafluoroethylene liner. After airtight, they were statically crystallized at room temperature for 24 hours. After the reaction is finished, it is centrifuged and washed to neutrality, and freeze-dried to obtain the -LDH precursor of the nanocatalyst.
[0035] Take 0.5g of NiFe-LDH, 0.19g of dicyandiamide and 1.31g of melamine and grind them thoroughly in a mortar until they are evenly mixed. -1 Raise the temperature to 500°C and keep it warm for 6h, and get th...
Embodiment 3
[0038] 4.04gFe(NO 3 ) 3 9H 2 O, 11.632gNi(NO 3 ) 2 ·6H 2 O was dissolved in 100mL deionized water, ultrasonicated for 5min, mixed thoroughly, and recorded as solution A.
[0039] 2.56 g NaOH and 125 μL of 30% H 2 o 2 Dissolved in 100mL deionized water, ultrasonicated for 5min, mixed thoroughly, and recorded as solution B.
[0040] The two solutions were slowly mixed at room temperature and added to the colloid mill at a controlled speed of 3000rpm. After being vigorously stirred for 2 minutes, they were transferred to a polytetrafluoroethylene liner. After airtight, they were statically crystallized at room temperature for 24 hours. After the reaction is finished, it is centrifuged and washed to neutrality, and freeze-dried to obtain the LDH precursor of the nanocatalyst.
[0041] Take 0.5g NiFe-LDH, 0.19g dicyandiamide and 1.31g melamine and grind them thoroughly in a mortar until they are evenly mixed, place them in a porcelain boat, raise the temperature to 500°C at...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com