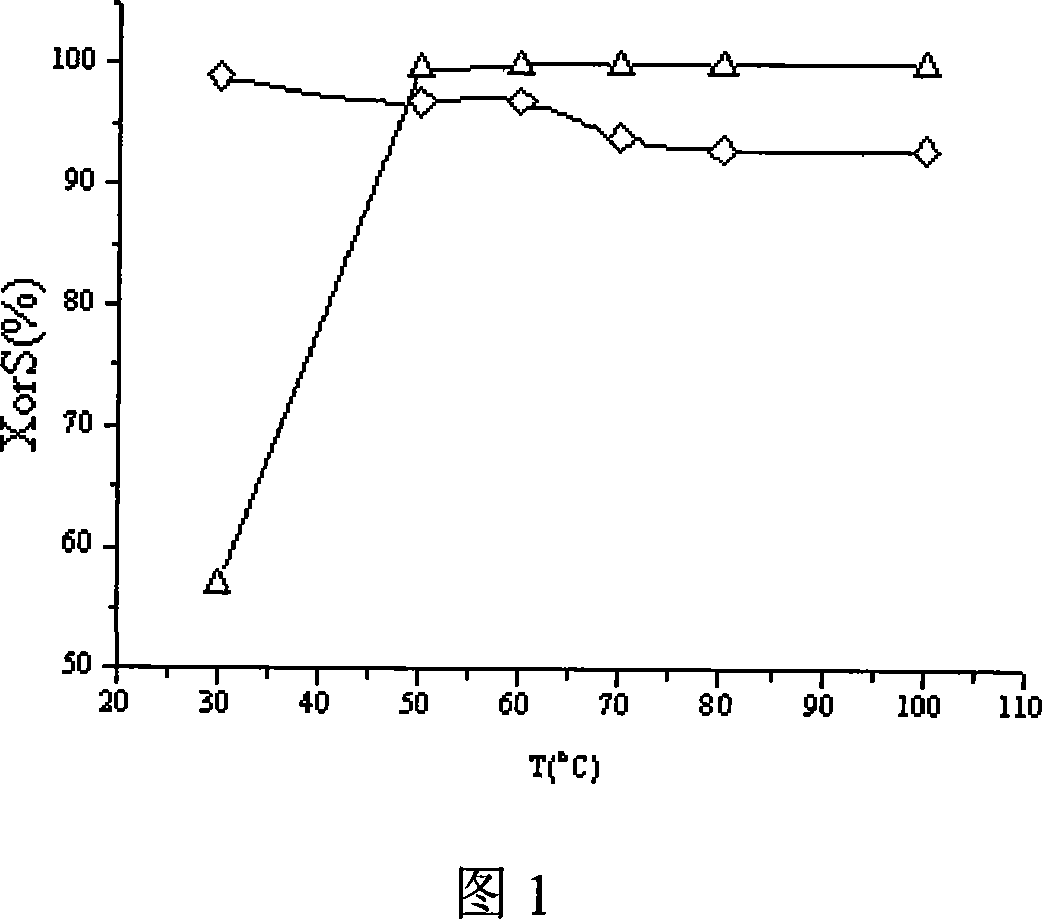
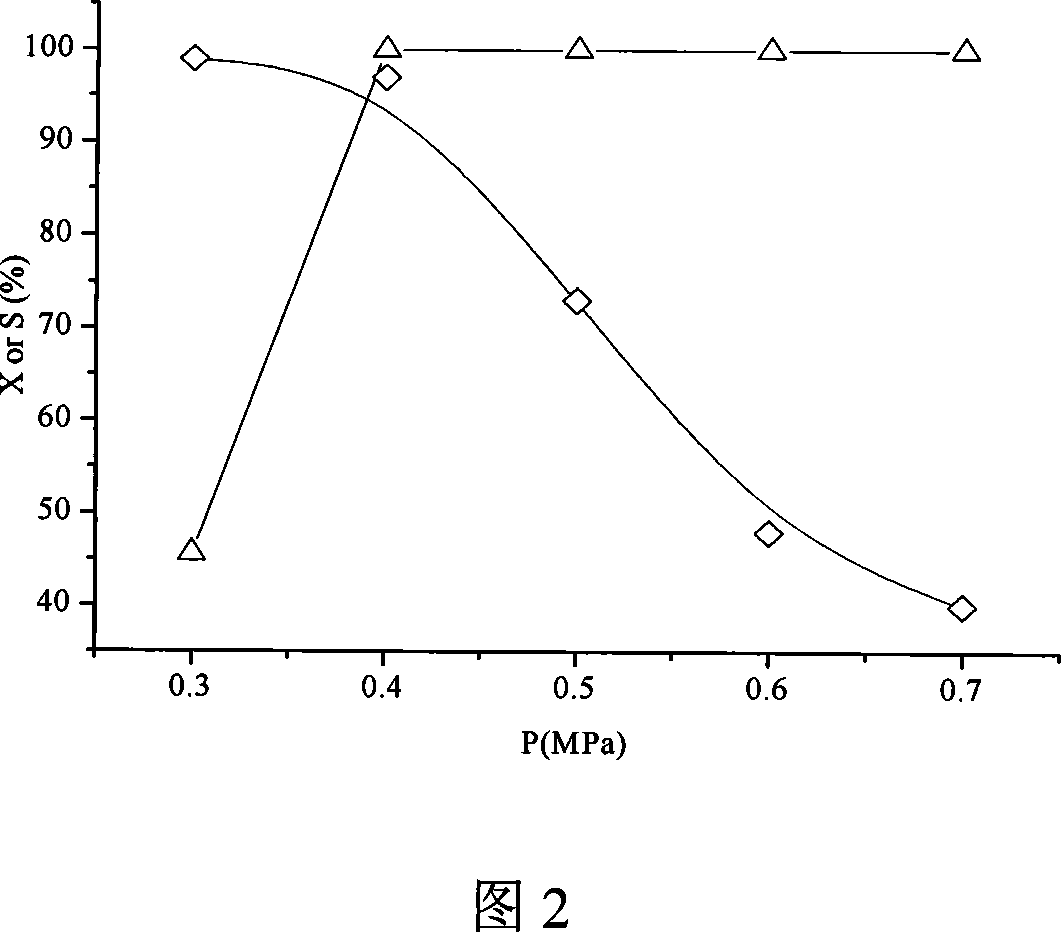
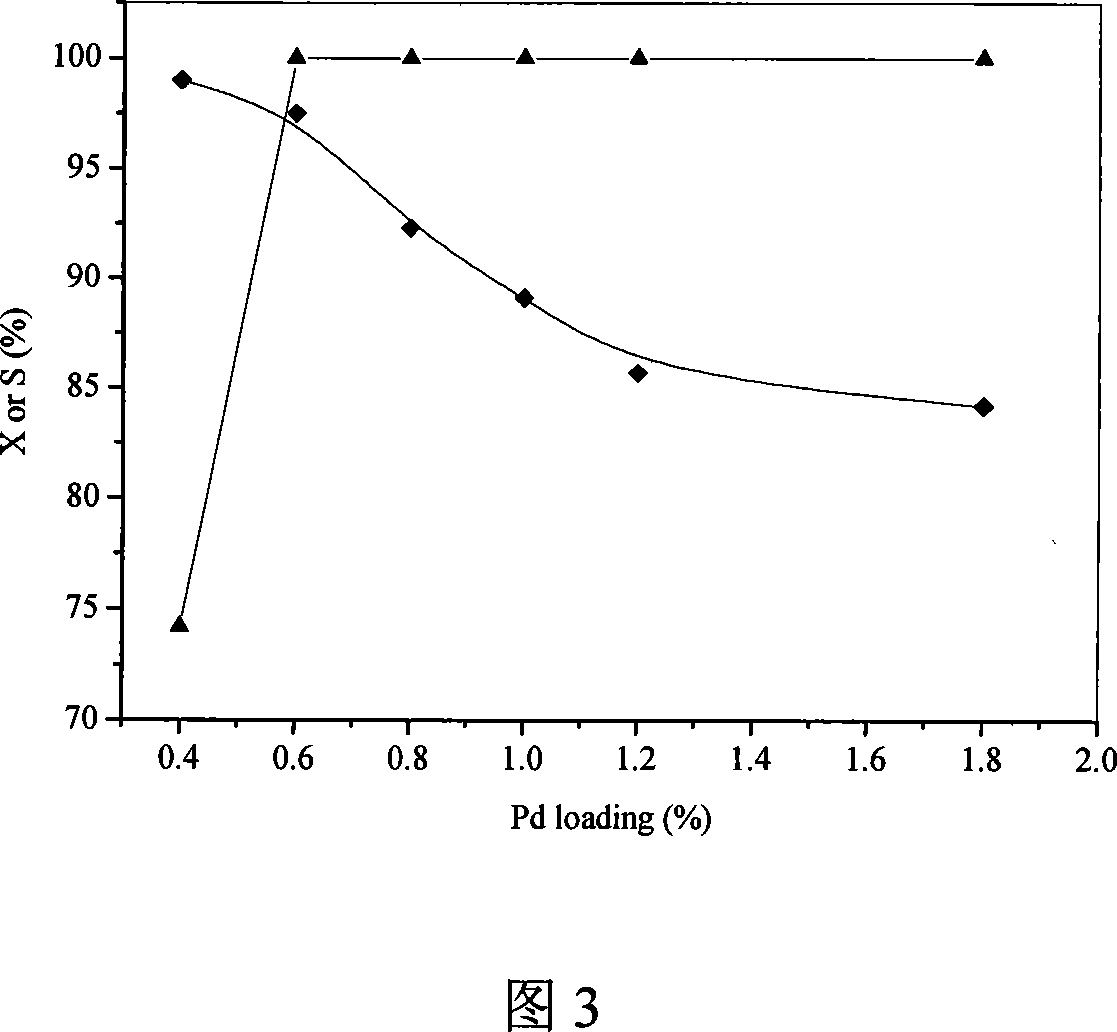
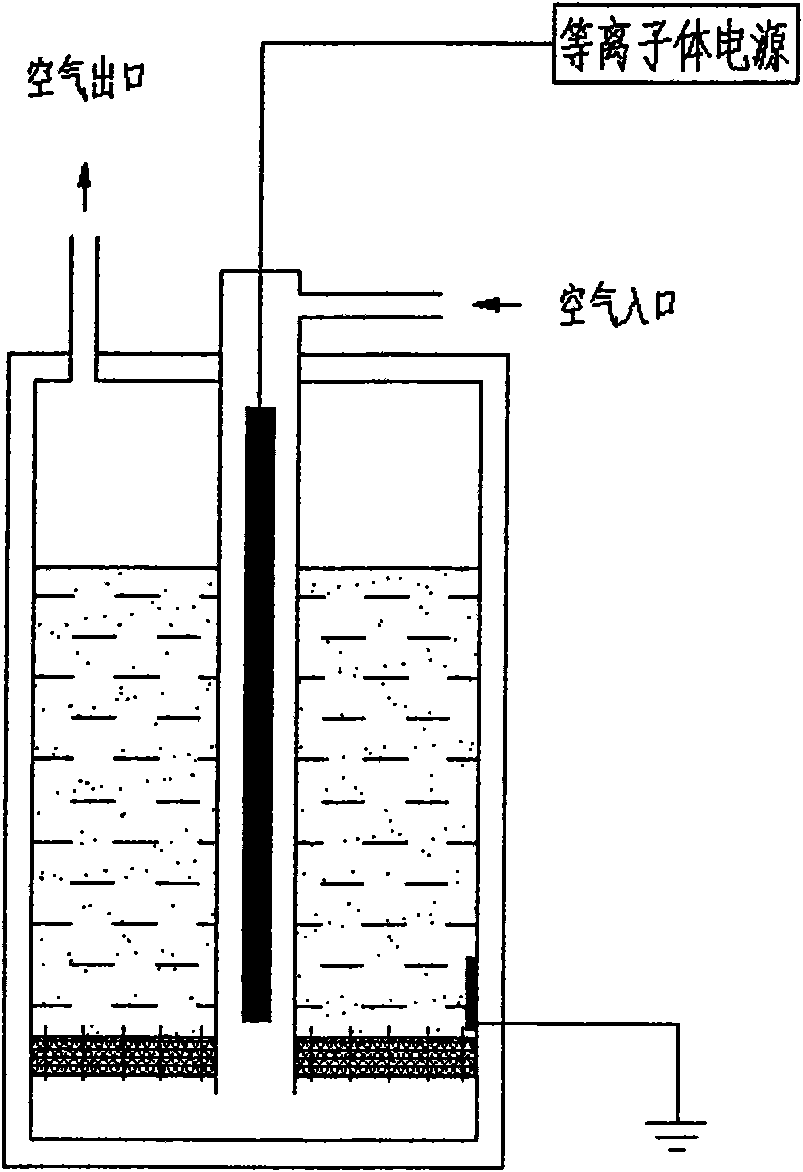
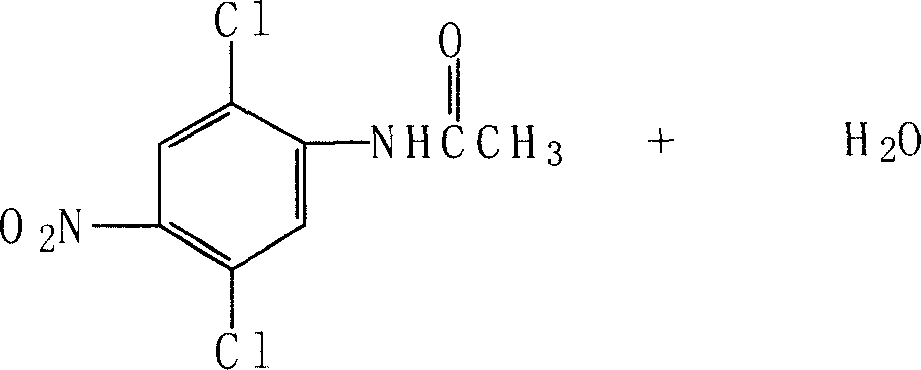
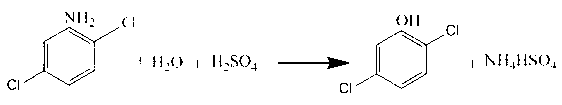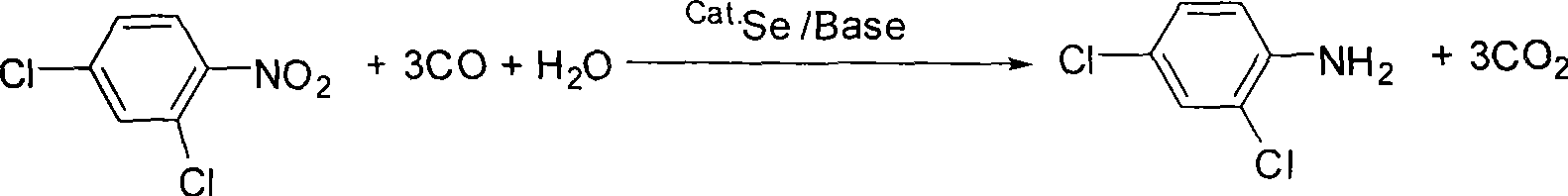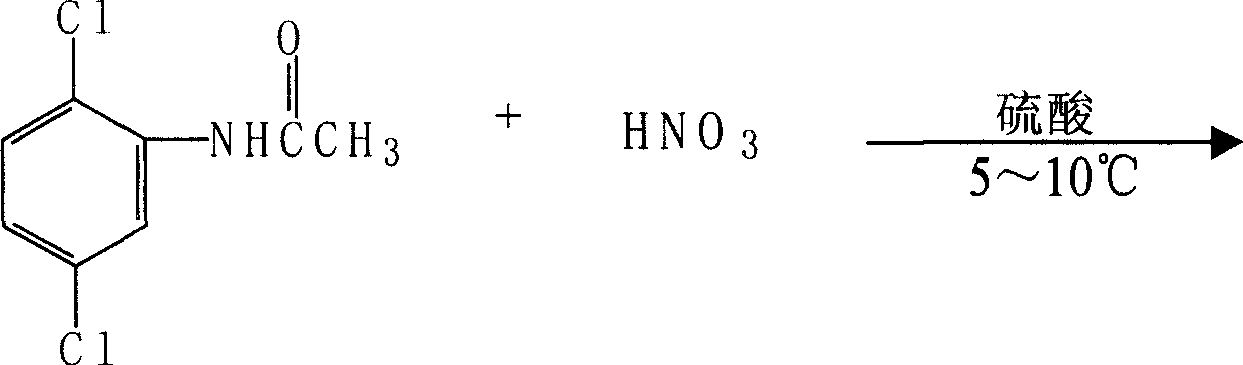Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
196 results about "Dichloroaniline" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
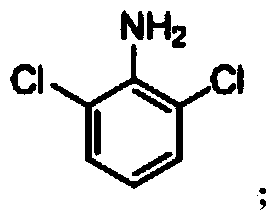
Dichloroanilines are chemical compounds which consist of an aniline ring substituted with two chlorine atoms and have the molecular formula C₆H₅Cl₂N. There are six isomers, varying in the positions of the chlorine atoms around the ring relative to the amino group. As aniline derivatives, they are named with the amino group in position 1. They are all colorless, although commercial samples can appear colored due to the presence of impurities. Several derivatives are used in the production of dyes and herbicides.
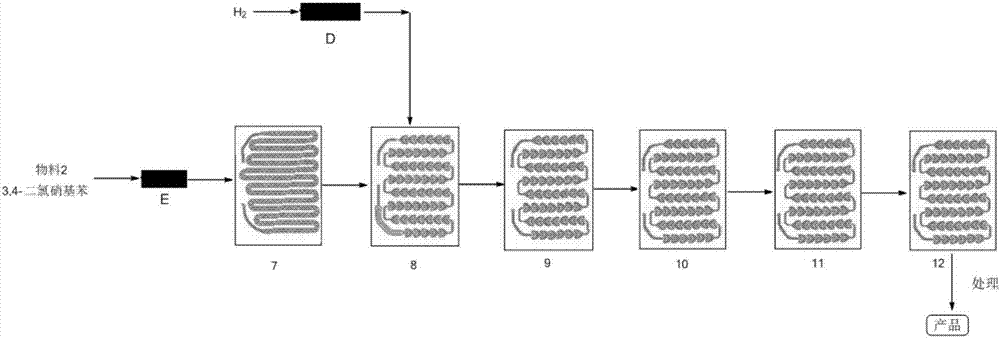
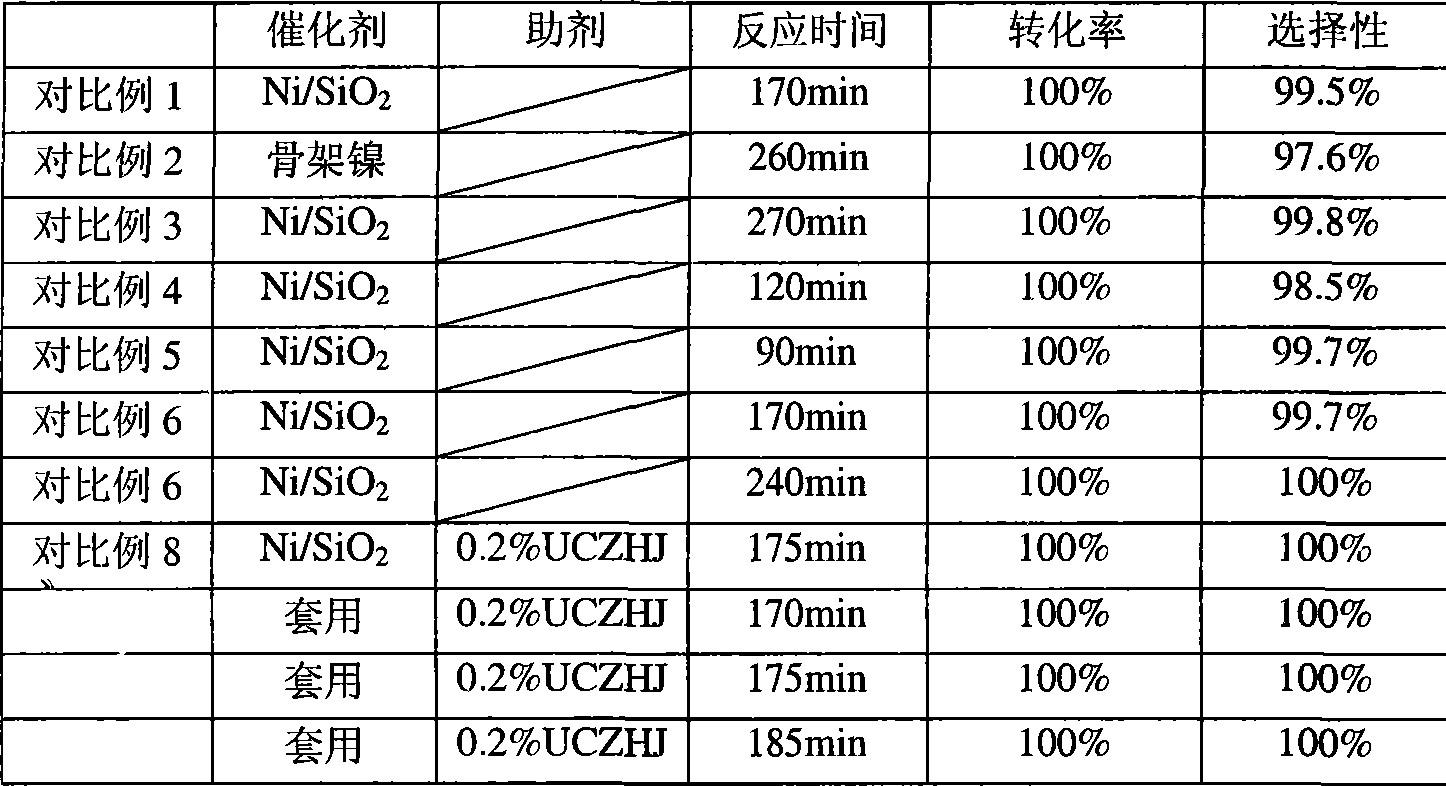
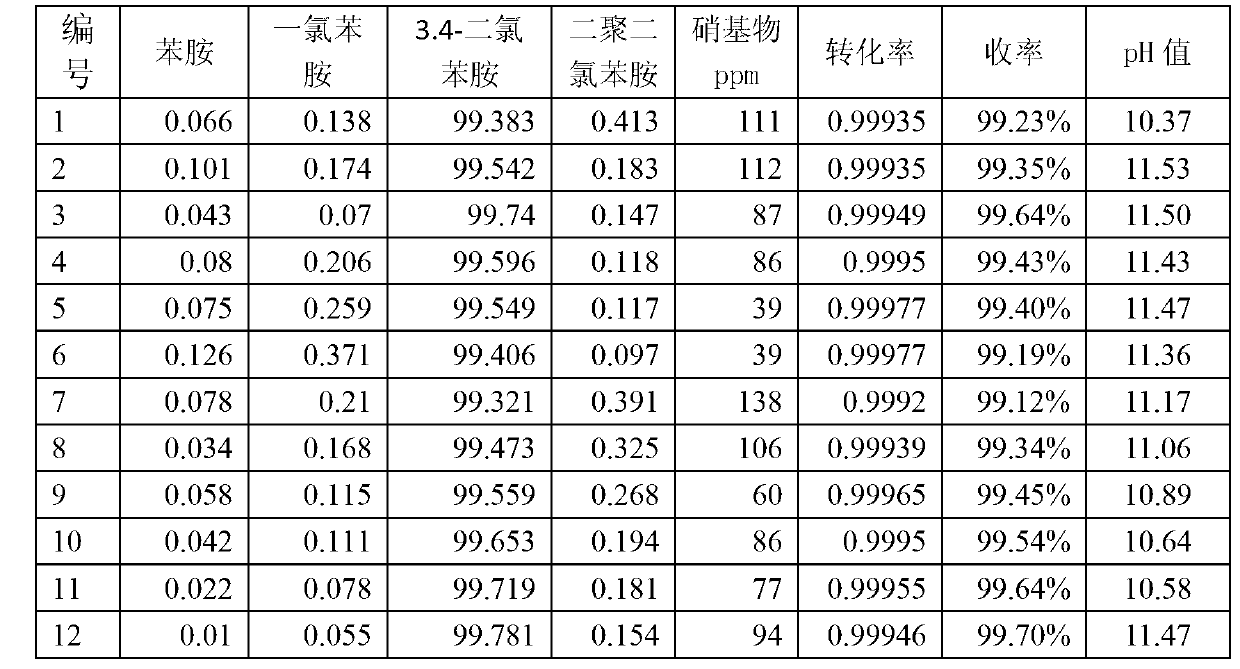
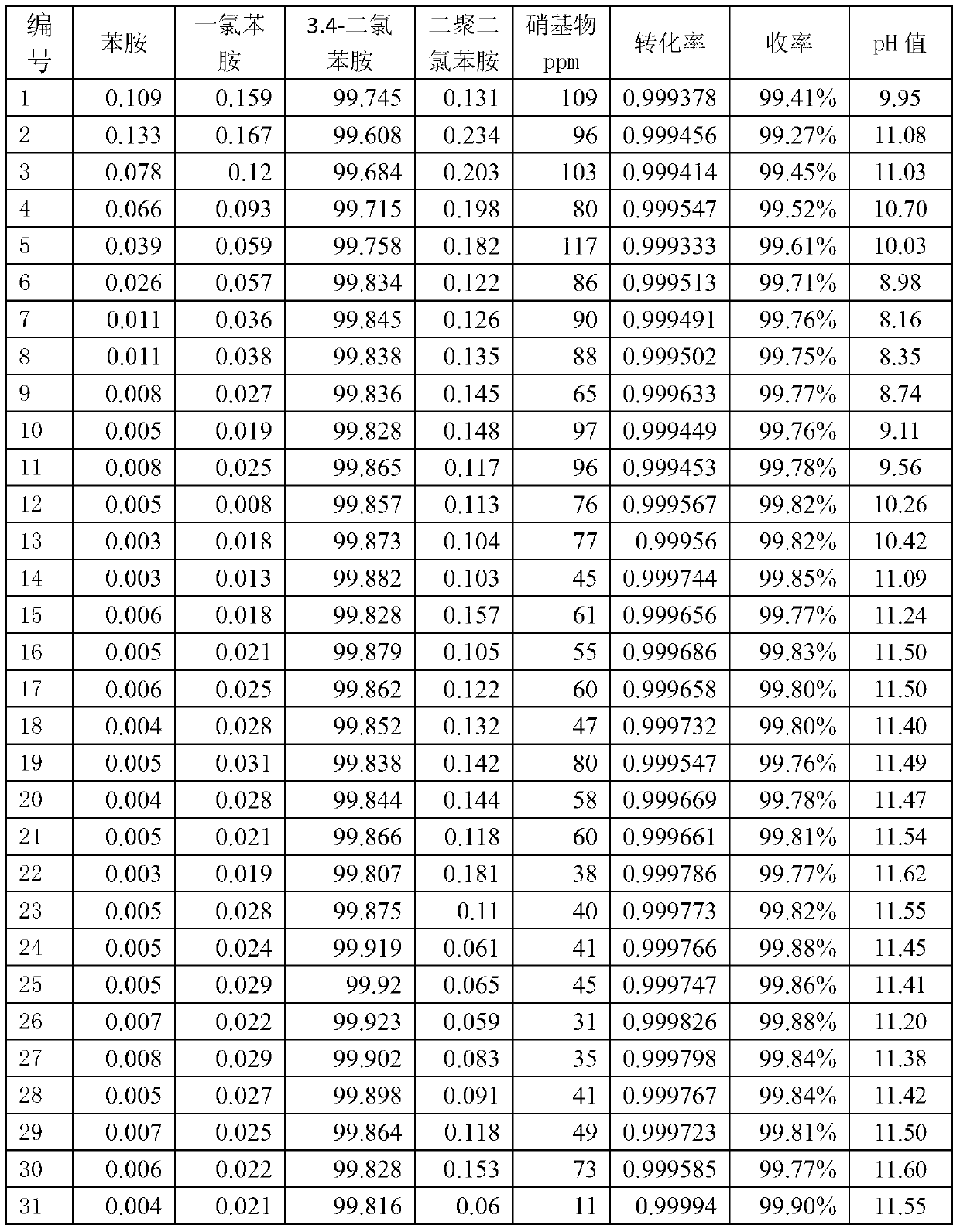
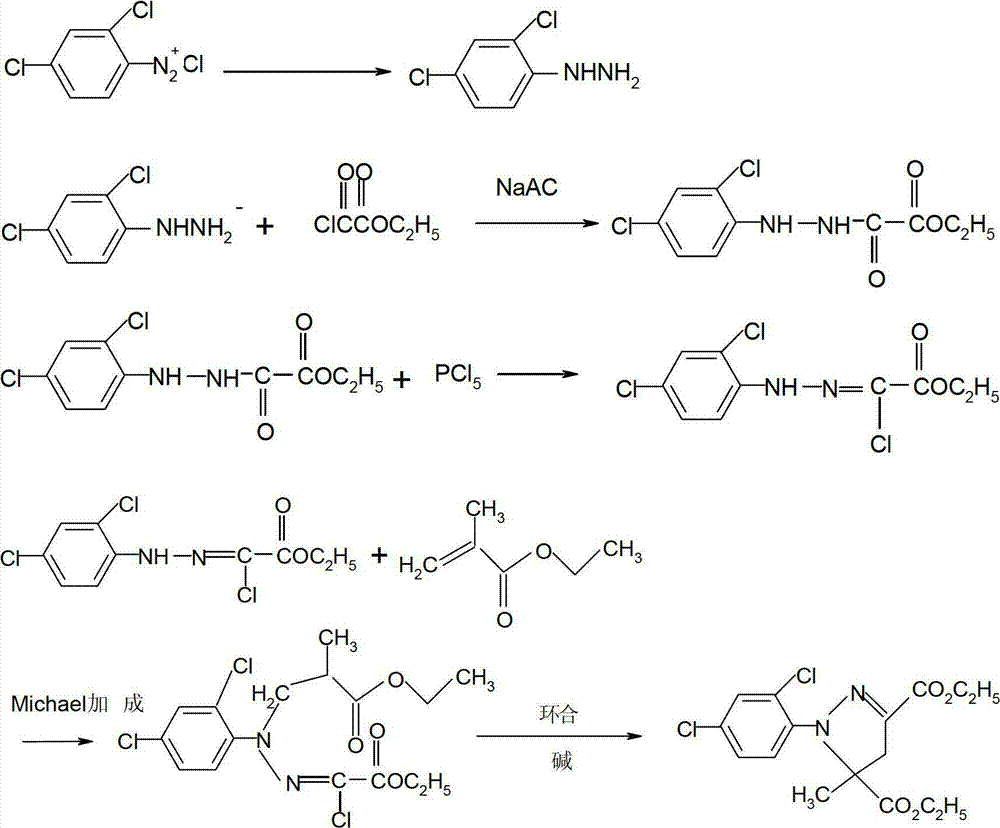
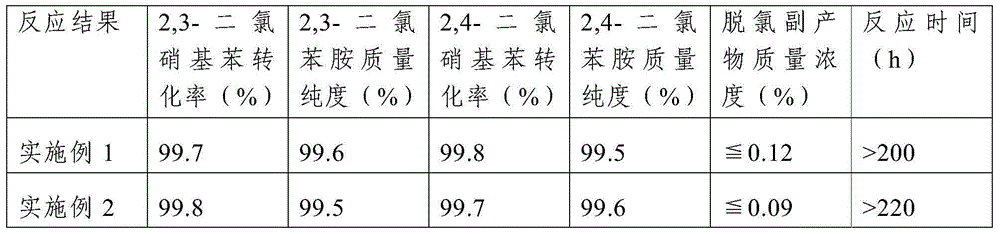
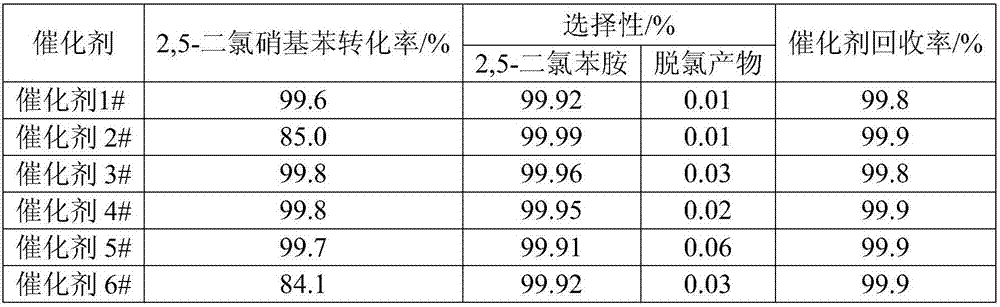
Production of 3,4-dichloroaniline catalyst with 3,4-mirbane oil dichloride hydrogenation
InactiveCN1817455AEasy to makeQuality is not affectedOrganic compound preparationCatalyst activation/preparationActivated carbonHalogen
A catalyst for preparing 3,4-dichlorophenylamine from 3,4-dichloronitrobenzene by hydrogenating is prepared through immersing activated carbon in the aqueous solution of potassium (or sodium) halide, washing until no halogen ions, immersing it in the aqueous solution of H2PdCl4, regulating pH value, filtering, washing filtered cake, reducing to obtain neutral product, and drying. It has high catalytic activity and selectivity.
Owner:ZHEJIANG UNIV OF TECH
Catalyst for preparing dichloroaniline through hydrogenization for dichloronitrobenzene, and preparation method
InactiveCN101049560ALow Pd contentApply many timesOrganic compound preparationAmino compound preparationActivated carbonActive component
A catalyst for preparing dichlorophenylamine by hydrogenating dichloronitrobenzene is composed of the activated carbon as carrier and the Pd as active component (0.2-2.0 Wt%) carried by said activated carbon. Its preparing process includes such steps as cyclically immersing activated carbon in diluted nitric acid, water washing until it becomes neutral, immersing in the aqueous solution of PdCl2, filtering, and baking the filtered cake. It has high catalytic performance and long cyclic service life.
Owner:EAST CHINA UNIV OF SCI & TECH
Production method for preparing chlorinated aniline via chlorination of nitrobenzene hydrogenation by utilizing solvent-free process
InactiveCN103113233ALow dechlorinationReduce addOrganic compound preparationAmino compound preparationSolvent freeNitrobenzene
The invention belongs to the production field of catalytic hydrogenation, and particularly discloses a production method for preparing chlorinated aniline via the chlorination of nitrobenzene hydrogenation by utilizing a solvent-free process. Chlorinated nitrobenzene is used as a raw material. The method is characterized in that under the existence of a catalyst and an aiding agent, the chlorinated nitrobenzene reacts with hydrogen at the temperature of 80 to 100 DEG C and under the pressure of 0.3 to 2.5 MPa, wherein a solvent is not added; and after the reaction is finished, the water diversion treatment is carried out, thereby obtaining the chlorinated aniline. The catalyst containing 1% of Pt / C precious metal is developed independently, wherein the adding amount of the catalyst accounts for 0.05% to 20% of that of the chlorinated nitrobenzene as the raw material. The aiding agent is a mixture of ethanol amine and pyridine, wherein the adding amount of the aiding agent accounts for 0.01% to 10% of that of the chlorinated nitrobenzene as the raw material. For the obtained o-chloroaniline, the chromatographic purity is above 99.5%, and the dechlorination quantity can be controlled within 0.1%. For the obtained 2,5-dichloroaniline, the chromatographic purity is above 99%, and the dechlorination quantity can be controlled within 0.1%. For the obtained 3,4-dichloroaniline, the chromatographic purity is above 99%, and the dechlorination quantity can be controlled within 0.1%.
Owner:SHANDONG FUYUAN CHEM
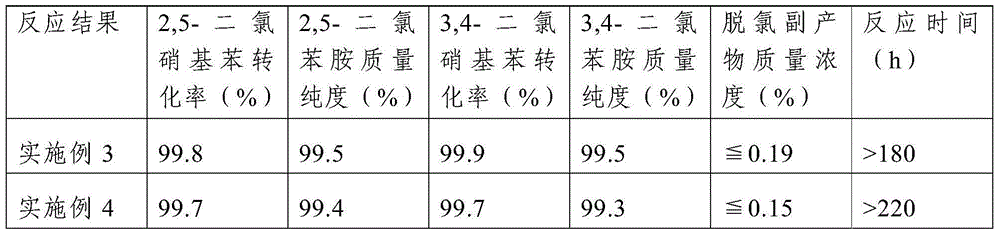
Method for processing 3, 4-dichloroaniline in water
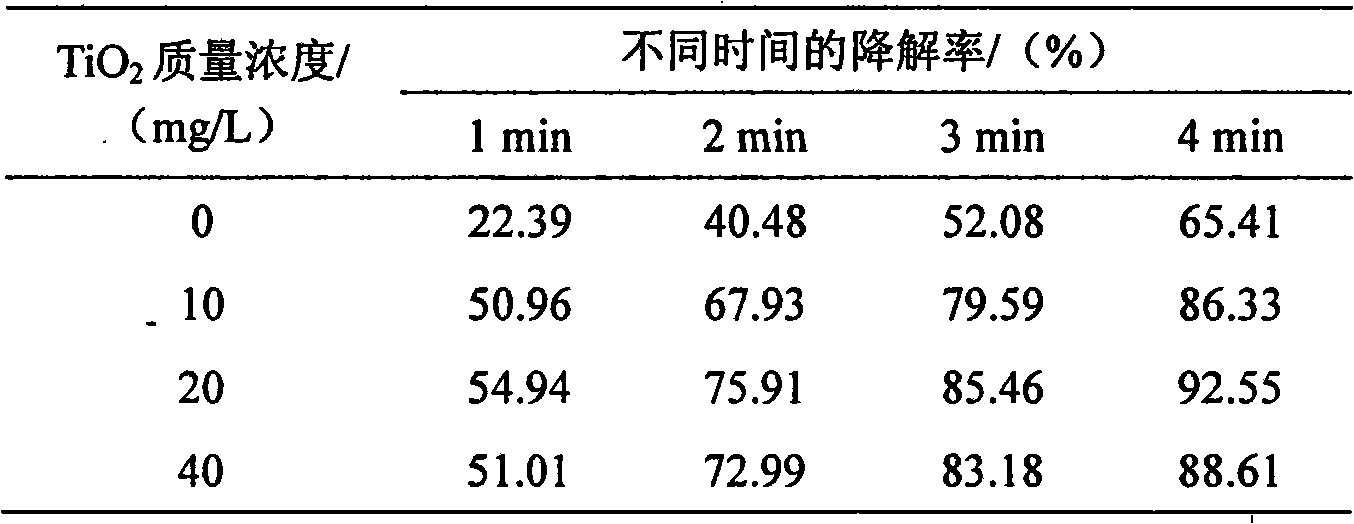
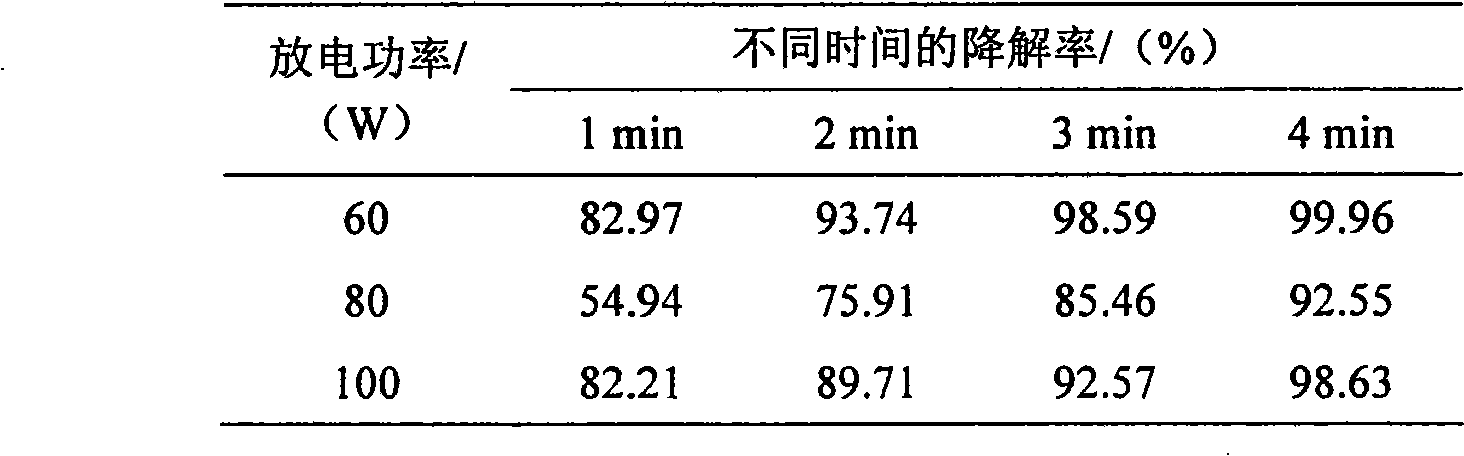
InactiveCN101559996AImprove photochemical reaction efficiencyImprove degradation efficiencyWater/sewage treatment by irradiationWater contaminantsResistLow voltage
The invention discloses a method for processing 3, 4-dichloroaniline in water. A high voltage electrode and a low voltage electrode are put in water body containing 3, 4-dichloroaniline to form a medium for resisting discharged electrodes, wherein the low voltage electrode contacts with water body to jointly form an earth electrode, the high voltage electrode connected with a power supply is separated from the water body by a medium of glass, air is introduced between the high voltage electrode and the medium of glass to form the medium for resisting discharge, thereby treating the water body; photocatalyst of titanium dioxide is added to the water body before the medium resists discharge, and ultraviolet light generated by plasma and photocatalyst can generate unique 'synergistic effect', thereby greatly improving degradation efficiency. The invention can effectively remove 3, 4-dichloroaniline component with biological toxicity in waste water for removing aniline. Compared with other plasma methods for resisting discharge by media, the invention has high degradation efficiency, short treatment time, small energy consumption, and simple and convenient devices of reactors and operation, etc.
Owner:NANJING UNIV
Catalytic hydrogenation method for preparing 3,4-dichloroaniline
InactiveCN1962608AHigh reactivityHigh selectivityOrganic compound preparationAmino compound preparationAlcoholDichloroaniline
The invention discloses a preparing method of 3, 4-dichloroaniline, which is characterized by the following: adopting 3, 4-dichloronitrobenzene as raw material and alcohol solution as solvent system; aerating H2 under 0.5-1.5Mpa at 80-120 Deg C in the condition of Raney-Ni catalyst and dechlorination inhibitor; proceeding catalytic hydrogenating reducing reaction to produce the product. The selectivity of 3, 4-dichloroaniline is more than 99%, whose dechlorination quantity is less than 2%.
Owner:ZHONGBEI UNIV
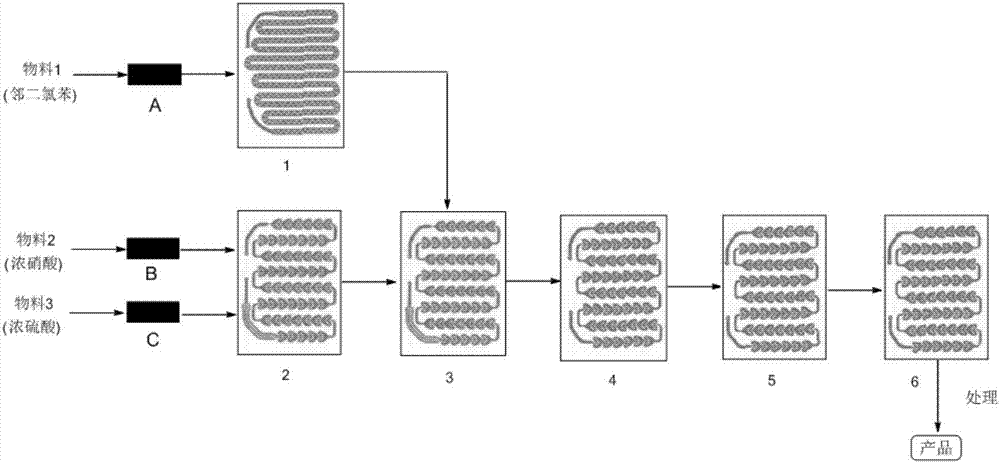
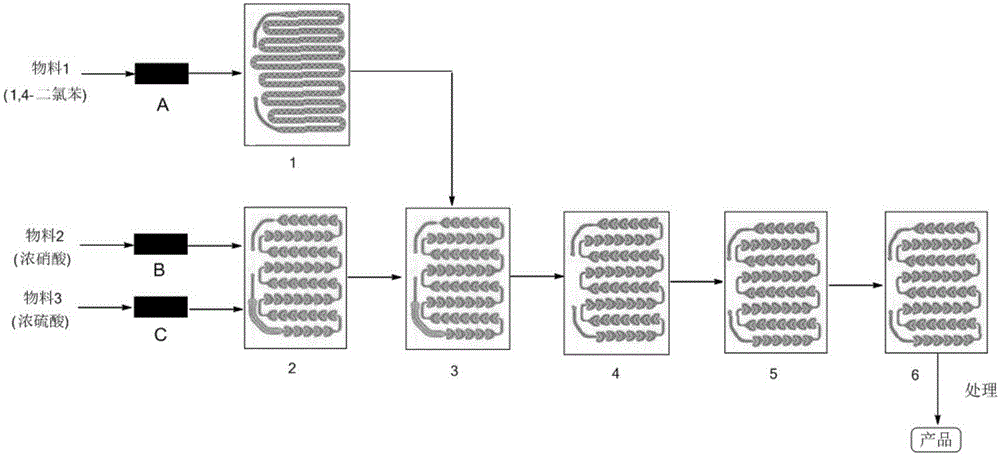
Method for synthesizing 3,4-dichloroaniline by using micro-channel reactor
InactiveCN107973720AIncrease contentLocal temperature guaranteeOrganic compound preparationAmino compound preparationHydrogenNitration
The invention provides a method for synthesizing 3,4-dichloroaniline by using a micro-channel reactor. The micro-channel reactor is used for nitration and catalytic hydrogenation. The method comprisesthe following steps: nitration: preheating o-dichlorobenzene, mixing and preheating concentrated nitric acid and concentrated sulfuric acid, allowing the preheated materials to enter a reaction module group for a reaction and carrying out refining so as to obtain an intermediate 3,4-dichloronitrobenzene; and catalytic hydrogenation: dissolving 3,4-dichloronitrobenzene in a solvent, adding a Pd-loaded active carbon catalyst, adding a dechlorination inhibitor, then carrying out preheating, allowing a preheated product and hydrogen to enter a reaction module group for a reaction and carrying outpost-treatment so as to obtain 3,4-dichloroaniline. The method provided by the invention is good in material mixing effect, accurate in material proportion control, capable of improving reaction yield and product purity, safe and stable in reactions, short in synthesis time and free of amplification effect, and has good application prospects in industrial production.
Owner:HEILONGJIANG XINCHUANG BIOLOGICAL TECH DEV CO LTD
Papermaking shoe press belt
ActiveUS20110017419A1Excellent especially in concave groove-shape retaining propertyGood shape retentionPretreated surfacesPaper/cardboardFiberMethylaniline
A papermaking shoe press belt is formed of a reinforcing fiber base material and a polyurethane layer integrated with each other. The reinforcing fiber base material is embedded in the polyurethane layer. The papermaking shoe press belt includes, as the polyurethane layer, a polyurethane layer obtainable by curing a composition composed in combination of a urethane prepolymer and one or more curing agent. The urethane prepolymer is obtainable by reacting a p-phenylene diisocyanate compound with a long-chain polyol. The at least one curing agent is selected from 4,4′-methylene bis(2,6-diethyl-3-chloroaniline), 4,4′-methylene bis(2-chloroaniline), methylene bis(2-ethyl-6-methylaniline), 4,4′-methylene bis(2-ethylbenzeneamine), methylene bis(2,3-dichloroaniline), 4,4′-methylenedianiline, 3,5-dimethylthiotoluene-2,4-diamine, 3,5-dimethylthiotoluene-2,6-diamine, 3,5-diethyltoluene-2,4-diamine, 3,5-diethyltoluene-2,6-diamine, polytetramethylene oxide di-p-aminobenzoate, poly(tetramethylene / 3-methyl tetramethylene ether)glycol bis(4-aminobenzoate), trimethylene bis(4-aminobenzoate) and isobutyl 4-chloro-3,5-diaminobenzoate.
Owner:ICHIKAWA ELECTRIC CO LTD
Preparation of supported nanometer nickel catalyst and application thereof
The invention relates to a supported nanometer nickel catalyst, which adopts isopyknic impregnation method and loads active metal components on the carrier, with the active metal content from 0.1 percent to 5 percent. And then the active metal induces the metal nickel in nickel and phosphorus bath to deposit to yield high activity supported nanometer nickel catalyst with 15 percent content of active component nickel. The catalyst used to prepare 2, 5-dichloroaniline from chloronitrophen added with hydrogen yields high hydrogen activity. Especially when UCZHJ is used a reaction promoter, the reaction yield reaches 100 percent, and the catalyst can be used repeatedly.
Owner:天津市瑞凯科技发展有限公司 +1
Production method of 3,4-dichloro aniline
InactiveCN1724508AHigh yieldLow costOrganic compound preparationAmino compound preparationAlcoholHydrogen
The invention provides a process for preparing 3,4-dichloroaniline, which comprises using 3,4-dichloro nitrobenzene as raw material, carrying out reduction reaction at 130-180 deg. C at the presence of alcoholic solution and catalyst, wherein the catalyst is Ru-Fe / Al203, and the amount of the catalyst is 1-40 wt% of 3,4-dichloronitrobenzene. The invention realizes high yield and low making cost, and dehydrogenation can be inhibited effectively.
Owner:ZHEJIANG UNIV OF TECH
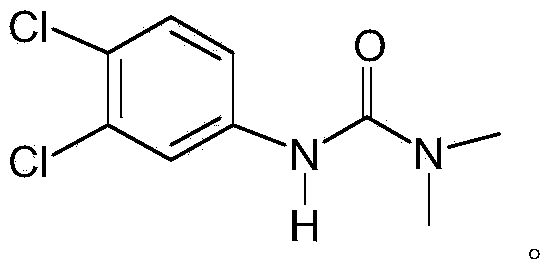
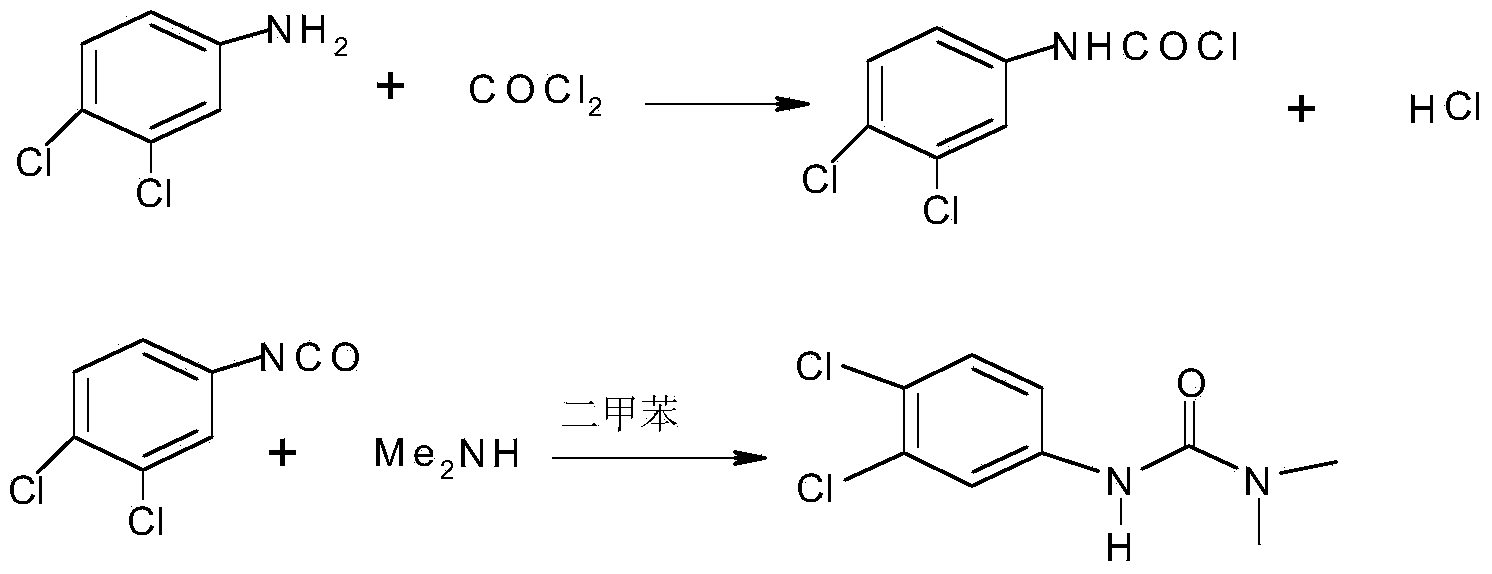
Method for preparing diuron
InactiveCN103539704AReduce generationAvoid decompositionUrea derivatives preparationOrganic compound preparationDecompositionWastewater
The invention discloses a method for preparing diuron. The method comprises the steps of firstly forming 3, 4-dichlorobenzene isocyanate through a reaction of 3, 4-dichloroaniline and phosgene, directly reacting the 3, 4-dichlorobenzene isocyanate with dimethylamine gas and finally preparing the diuron. The method disclosed by the invention has the advantages that the dimethylamine gas is directly introduced into an organic phase to react and a traditional dimethylamine aqueous solution is replaced, so that the decomposition of the 3, 4-dichlorobenzene isocyanate in an aqueous phase is avoided, the forming of by-products is reduced, the cost is saved, wastewater is not produced and environmental protection is benefitted; the purity of the prepared diuron is more than 99% and the total recovery of the 3, 4-dichloroaniline is more than 95%.
Owner:JIANGSU ANPON ELECTROCHEM
Method for preparing triclabendazole
ActiveCN101555231AReduce usageAvoid hyperbaric reactionsOrganic chemistryChemical synthesisTriclabendazole
The invention discloses a method for preparing triclabendazole related to chemical synthesis methods. The invention comprises the following steps: using the 3,4-dichloroaniline as a raw material, preparing 4-chloro-5-(2,3-dichlorophenoxy)-2-mercapto-1H-benzimidazole through acylating, nitrating, hydrolyzing, etherifying, reduction and cyclization in succession and synthesizing triclabendazole using dimethyl sulfate as methylating agent. The invention avoids the use of the hazardous material sodium and a high pressure reaction, uses easily available dimethyl sulfate as methylating agent, and the yield thereof is equivalent to the report of literature. The invention is characterized by simplified work, moderate reaction condition, cheap production cost and the method is also environmentally-benign and easy to commercial process.
Owner:YANGZHOU TIANHE PHARM CO LTD
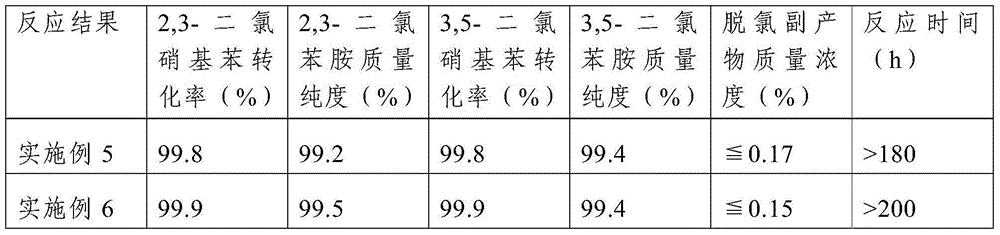
Method for high-selectivity preparation of 3,4-dichloroaniline
ActiveCN103694124AReduce corrosionSolve the problem of hydrogenolysis and dechlorinationOrganic compound preparationAmino compound preparationPtru catalystNitrobenzene
The invention belongs to the technical field of fine chemical engineering, and relates to a method for preparing 3,4-dichloroaniline from 3,4-dichloronitrobenzene through high-selectivity catalytic hydrogenation. With 3,4-dichloronitrobenzene as raw material and in the presence of a Pt catalyst, 3,4-dichloroaniline is prepared through a catalytic hydrogenation reaction under the pressure of 1.0 MPa-3.0 MPa and at the temperature of 75-120 DEG C. With adoption of the method, the conversion rate of 3,4-dichloronitrobenzene is 100%, the selectivity of 3,4-dichloroaniline is greater than 99.6%, and the dechlorination rate is less than 0.20%. The Pt catalyst is safe to use, and has stable catalytic activity and high selectivity; a dechlorination inhibitor is not required to be added; through introduction of Fe2O3 into the Pt catalyst, a dechlorination phenomenon can be effectively inhibited, and the corrosion of dechlorination to a reaction container during reduction is reduced; and the method is not added with a solvent, overcomes the defect of the addition of the solvent, avoids the problems of pollution of the solvent to the environment and solvent recovery, reduces equipment investment, and reduces production costs.
Owner:CHINA PETROLEUM & CHEM CORP +1
Method for continuously producing 3,4-dichloroaniline
InactiveCN103342650AStable process parametersSimple control methodOrganic compound preparationAmino compound preparationNickel catalystHydrogen
The invention relates to a method for continuously producing 3,4-dichloroaniline. The method comprises the following steps of: continuously putting a 3,4-dichloronitrobenzene solution into a stirred tank reactor, wherein the pressure of the stirred tank reactor is 0.5-2Mpa, the temperature is 60-120 DEG C, and the pH value in a stirred tank is controlled to be between 8 and 12; performing catalytic hydrogenation reaction on the 3,4-dichloronitrobenzene in the stirred tank reactor in the presence of a nickel catalyst, a dechlorination inhibitor, hydrogen and a solvent to generate 3,4-dichloroaniline; overflowing the reaction product into a tilted plate separator, and continuously separating a catalyst concentrated solution and a product clear solution in the tilted plate separator; and continuously returning the catalyst concentrated solution to the stirred tank reactor. According to the method, the yield of the product obtained by the method is more than 99%, the dechlorination mass fraction is less than 0.5%, the loss of the catalyst is low, the consumption of the hydrogen is small, and the operation is stable and easy to control, so that the method is suitable for industrial large-scale production.
Owner:QINGDAO UNIV OF SCI & TECH +1
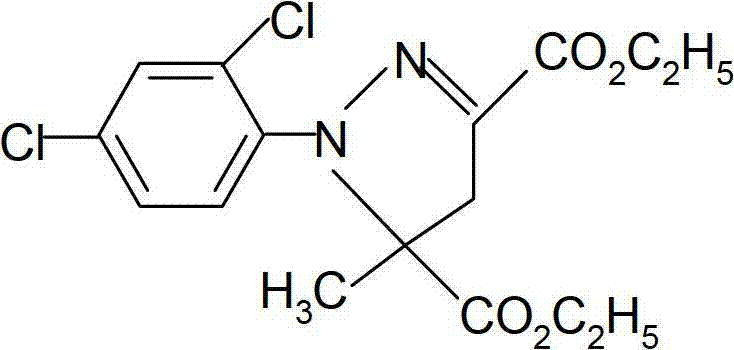
Catalytic synthesis method of mefenpyr-diethyl
The invention discloses a catalytic synthesis method of (RS)-1-(2,4-dichlorophenyl)-5-methyl-2-pyrazol-3,5-dicarboxylate, comprising the following steps of: A, diazotization: a diazo reaction is carried out by the use of 2,4-dichloroaniline, hydrochloric acid and sodium nitrite so as to obtain aniline diazo salt; B, synthesis of chloro-ethyl acetoacetate: chlorination is carried out by the use of ethyl acetoacetate and sulfuric chloride to obtain chloro-ethyl acetoacetate; C, synthesis of 2-chlorine-2-(2,4-dichlorophenylhydrazone)-ethyl glyoxalate: diazo salt obtained from the step A reacts with chloro-ethyl acetoacetate obtained from the step B under the action of a catalyst to obtain 2-chlorine-2-(2,4-dichlorophenylhydrazone)-ethyl glyoxalate; and D, cyclization: a cyclization reaction between 2-chlorine-2-(2,4-dichlorophenylhydrazone)-ethyl glyoxalate obtained from the step C and methyl methacrylate is carried out so as to obtain (RS)-1-(2,4-dichlorophenyl)-5-methyl-2-pyrazol-3,5-dicarboxylate. The method provided by the invention has advantages of simple technology, easily-obtained raw materials and high yield of the product.
Owner:JIANGSU TIANRONG GROUP
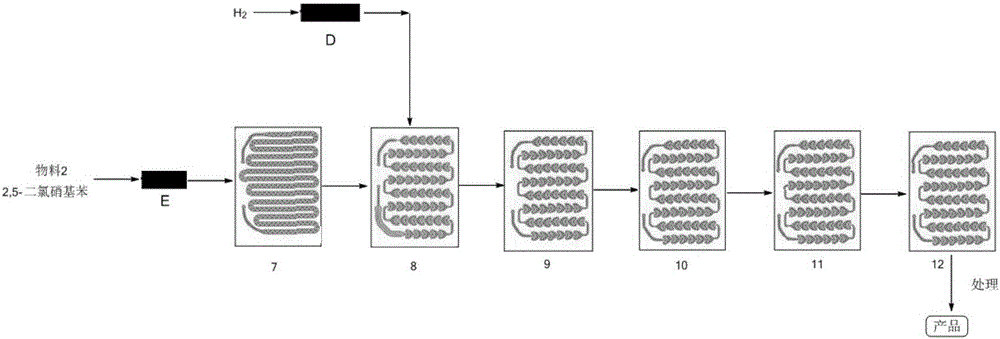
Method for synthesizing 2,5-dichloroaniline by micro-channel reactor
InactiveCN106565500AHigh energy consumptionInhibitory reactivityOrganic compound preparationChemical/physical/physico-chemical microreactorsNitrationSolvent
The invention provides a method for synthesizing 2,5-dichloroaniline by a micro-channel reactor. A nitratlon reaction and a catalytic hydrogenation reaction are performed by using the micro-channel reactor. The method comprises the following steps of nitratlon reaction: raw materials of nitro-p-dichlorobenzene are dissolved into an organic solvent, and are preheated; concentrated nitric acid and concentrated sulfuric acid are mixed and are preheated; the materials enter a reaction module group to take a reaction after the preheating; and an intermediate product of 2,5-Dichloronitrobenzene is obtained after the refining; and catalytic hydrogenation reaction: the 2,5-Dichloronitrobenzene is dissolved into a solvent; Pd-loaded active carbon catalysts are added; dechlorination inhibitors are added, and then, preheating is performed; after the materials are preheated, hydrogen gas enters the reaction module group to take a reaction; and post-treatment is performed to obtain the 2,5-dichloroaniline. The method provided by the invention has the advantages that the mixing effect is good; the temperature and material proportion control is precise; the reaction yield and the product purity are improved; the reaction is safe and stable; the time is short; no amplification effect exists; and wide application prospects are realized in industrial production.
Owner:HEILONGJIANG XINCHUANG BIOLOGICAL TECH DEV CO LTD
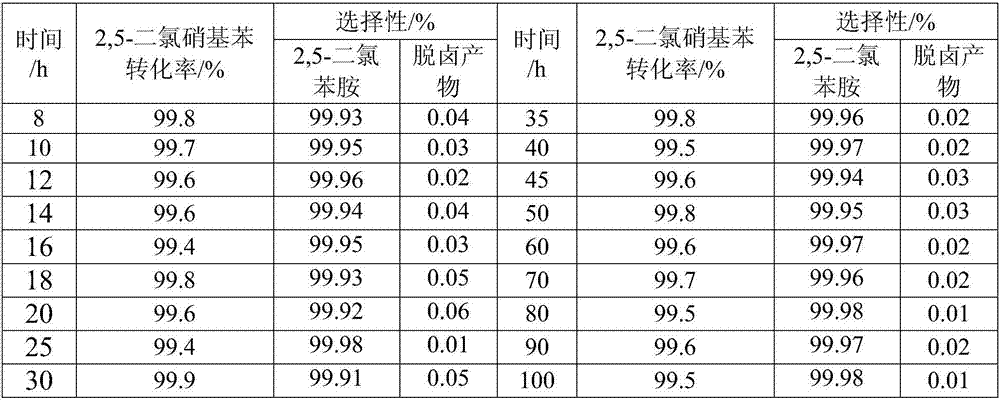
Method for preparing dichloroaniline by continuously catalyzing and hydrogenating dichloronitrobenzene
ActiveCN104672094AImprove performanceHigh catalytic hydrogenation reaction efficiencyOrganic compound preparationAmino compound preparationFixed bedReaction temperature
The invention discloses a method for preparing dichloroaniline by continuously catalyzing and hydrogenating dichloronitrobenzene. The method comprises the following steps: I, filling a fixed bed reactor with catalyst, and introducing reduction gas into the fixed bed reactor to reduce the catalyst; II, lowering the temperature of the fixed bed reactor to a reaction temperature, pumping an ammonia-ammonium chloride buffer solution, introducing the molten dichloronitrobenzene, and carrying out the catalytic hydrogenation reaction under the condition of the reaction temperature; and III, separating the material after the catalytic hydrogenation reaction by virtue of an oil-water separator to obtain an organic phase and a water phase, wherein the organic phase is the dichloroaniline. The conversion rate of the raw material dichloronitrobenzene is more than or equal to 99.5 percent, the mass concentration of a dechlorination byproduct is less than 0.2 percent, the mass purity of the dichloroaniline is more than or equal to 99.2 percent, the stability of the catalyst is stable, the catalytic hydrogenation reaction efficiency is high, and the process flow is capable of saving the resource and is environmentally friendly.
Owner:XIAN CATALYST NEW MATERIALS CO LTD
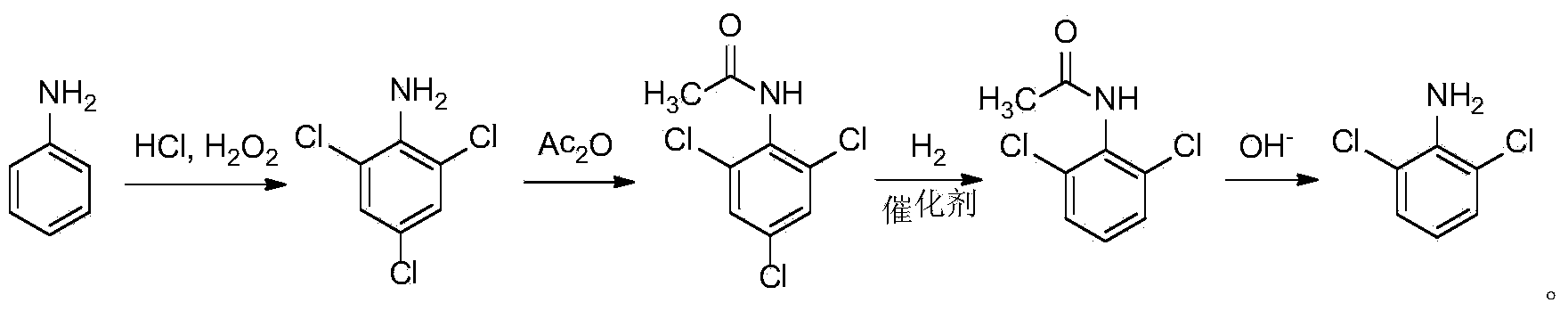
Synthetic method of 2, 6-dichloroaniline
InactiveCN103524358AReduce corrosionLess investmentOrganic compound preparationAmino compound preparationAcetic anhydrideHydrogen
The invention discloses a synthesis method of 2, 6-dichloroaniline. The method uses aniline as a raw material, and comprises the following steps: 1) adding aniline into dilute hydrochloric acid, then dropwise adding hydrogen peroxide for a chlorination reaction, and adding water into the obtained 2, 4, 6- trichloroaniline hydrochloride to carry out steam distillation, so as to obtain 2, 4, 6-trichloroaniline; 2) reacting 2, 4, 6-trichloroaniline with acetic anhydride to obtain 2, 4, 6-trichloroacetanilide; 3) adding the obtained 2, 4, 6-trichloroacetanilide into an autoclave, then adding a solvent and a catalyst, and filling in hydrogen for a reduction reaction, so as to obtain 2, 6-dichloroacetanilide; and 4) adding 2, 6-dichloroacetanilide into an alkali solution and heating for reaction, so as to finally obtain 2, 6-dichloroaniline. The synthetic method of 2, 6-dichloroaniline has the advantages of low cost, high conversion rate and simple post-treatment, and is suitable for industrial production.
Owner:ZHEJIANG UNIV
Synthesis method of 2, 5-dichlorophenol
InactiveCN102838457AReduce consumptionEmission reductionOrganic chemistryOrganic compound preparationDichlorophenolChlorophenol
The invention discloses a synthesis method of 2, 5-dichlorophenol, which comprises the following steps of: using 2, 5-dichloroaniline as raw materials and inorganic acid water solution as hydrolytic agent and solvent, firstly heating and premixing the 2, 5-dichloroaniline and the inorganic acid water solution at normal pressure, enabling the 2, 5-dichloroaniline to be fully dissolved in the inorganic acid water solution, then hydrolyzing the mixed solution in the existence of inert gas under conditions of high temperature and high pressure, reducing the temperature and the pressure of reaction solution after hydrolysis is completed, and statically placing the reaction solution for layering to separate out an organic phase which is the 2, 5-dichlorophenol. The synthesis method of 2, 5-dichlorophenol has the advantages that since diluted acid is used as the hydrolytic agent, the consumption of raw materials is reduced; and since the 2, 5-dichloroaniline is hydrolyzed through the high activity of the diluted acid solution at high temperature and high pressure to directly obtain the 2, 5-dichlorophenol, the conversion rate is high, the flow is short, the process is simple, the treatment method is simple after synthesis and the 2, 5-dichlorophenol can be well recovered.
Owner:SHANDONG WEIFANG RAINBOW CHEM
Polychlorinated biphenyl (PCBs) homologue semiantigen and preparation method thereof
InactiveCN101157611AOrganic compound preparationCarboxylic compound preparationWater bathsWater vapor
The invention relates to a PCBs homolog hapten and a preparation method thereof. The structural formula of PCBs hapten is right, wherein, R is equal to Cl, R1 is equal to H or Cl, n is equal to 1, 2, 3, R3 is equal to COCH2CH2COOH. The preparation method comprises the steps as follows: firstly, dichloroaniline is dissolved, thick salt is filled in for mixing, and the solution is heavily nitrided through 25 to 33 percent of sodium nitrate after being heated and dissolved through water of 20 to 40ml; secondly, 60 to 80ml of aromatic hydrocarbon solvent is filled in and mixed with the solution under 0 to 5 DEG C, and then thick alkali is dropped in the solution for mixing and reacting for 1 to 3 hours; thirdly, the reaction product is vaporized through vapor or the rude yellowish-brown product is obtained by extracting through non-polar organic solvent, 1 to 3g zinc powder and 1 to 3ml thick hydrochloric acid are filled in with no more gas generating and then mixture is filtered hotly and the crystal is extracted; fourthly, the mol rate of PCBs, succinic anhydride and water free AlCl3 is 1:1 to 2:1 to 3. The water free AlCl3 can be filled in multiple times within 1 to 2 hours under cold water bath. The reaction is stopped after being mixed for 16 to 56 hours in a sealing way. The invention has the advantages of simple step, quick and high yield.
Owner:SHANGHAI JIAO TONG UNIV
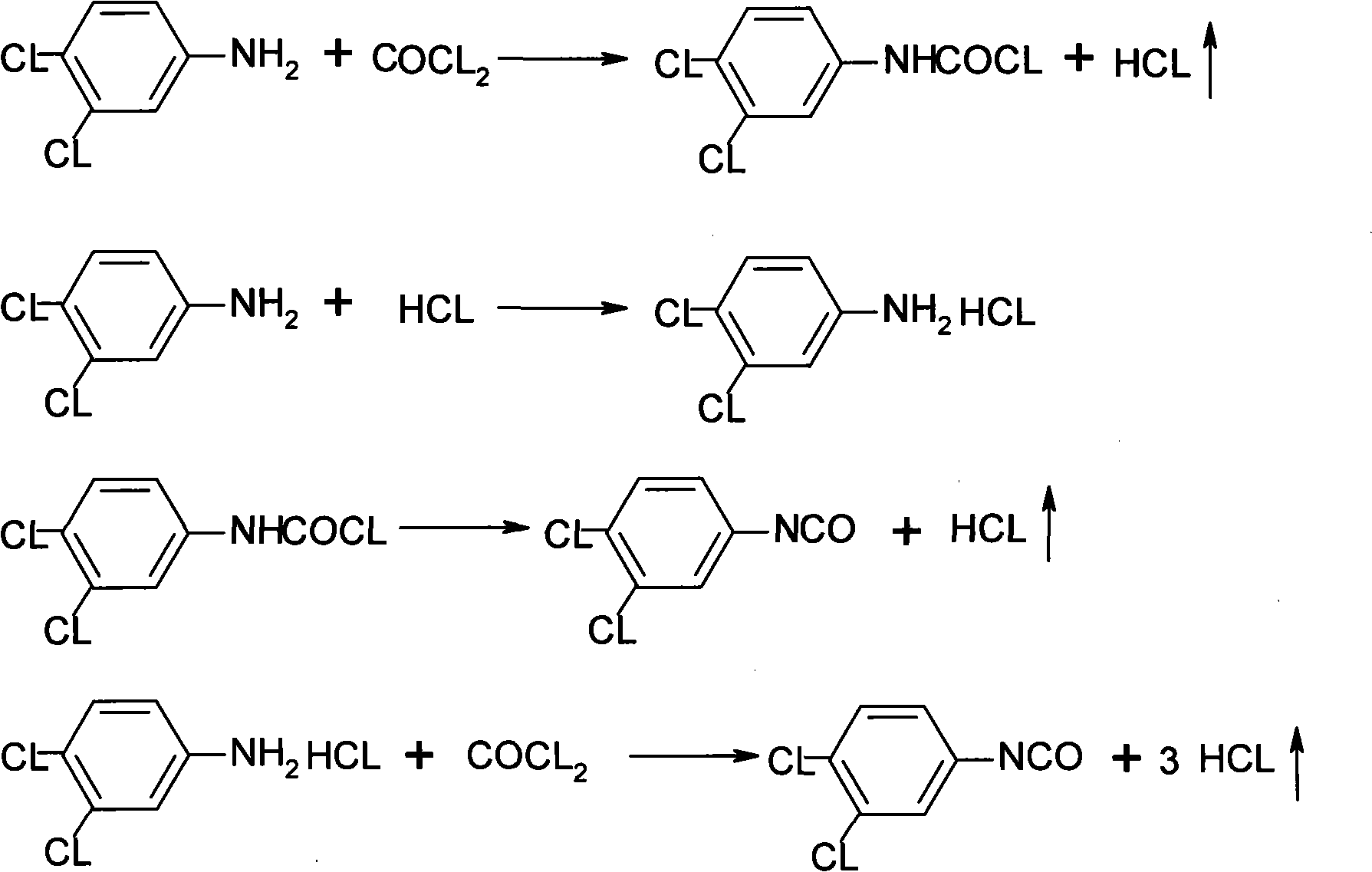
Preparation for 3, 4-dichloro phenyl isocyanate
ActiveCN101274904AReduce the temperatureLess side effectsIsocyanic acid derivatives preparationOrganic compound preparationChemical reactionOrganic solvent
The invention discloses a method for preparing 3, 4-dichloro phenyl isocyanate. The method takes 3, 4-dichloroaniline as raw material and xylene, or benzene chloride, o (p) dichlorobenzene and other organic solvents which do not react chemically with phosgene as a reaction solvent, goes through two steps of phosgenation reaction under low temperature and high temperature and distillation to obtain products with purity over 98 percent. The technique of two steps of phosgenation reaction under low temperature and high temperature can effectively reduce side reaction and improve reaction efficiency; the total yield of the invention achieves over 97 percent calculated by 3, 4-dichloroaniline.
Owner:JIANGSU ANPON ELECTROCHEM
Method and device for preparing 3,4-dichloroaniline without solvent
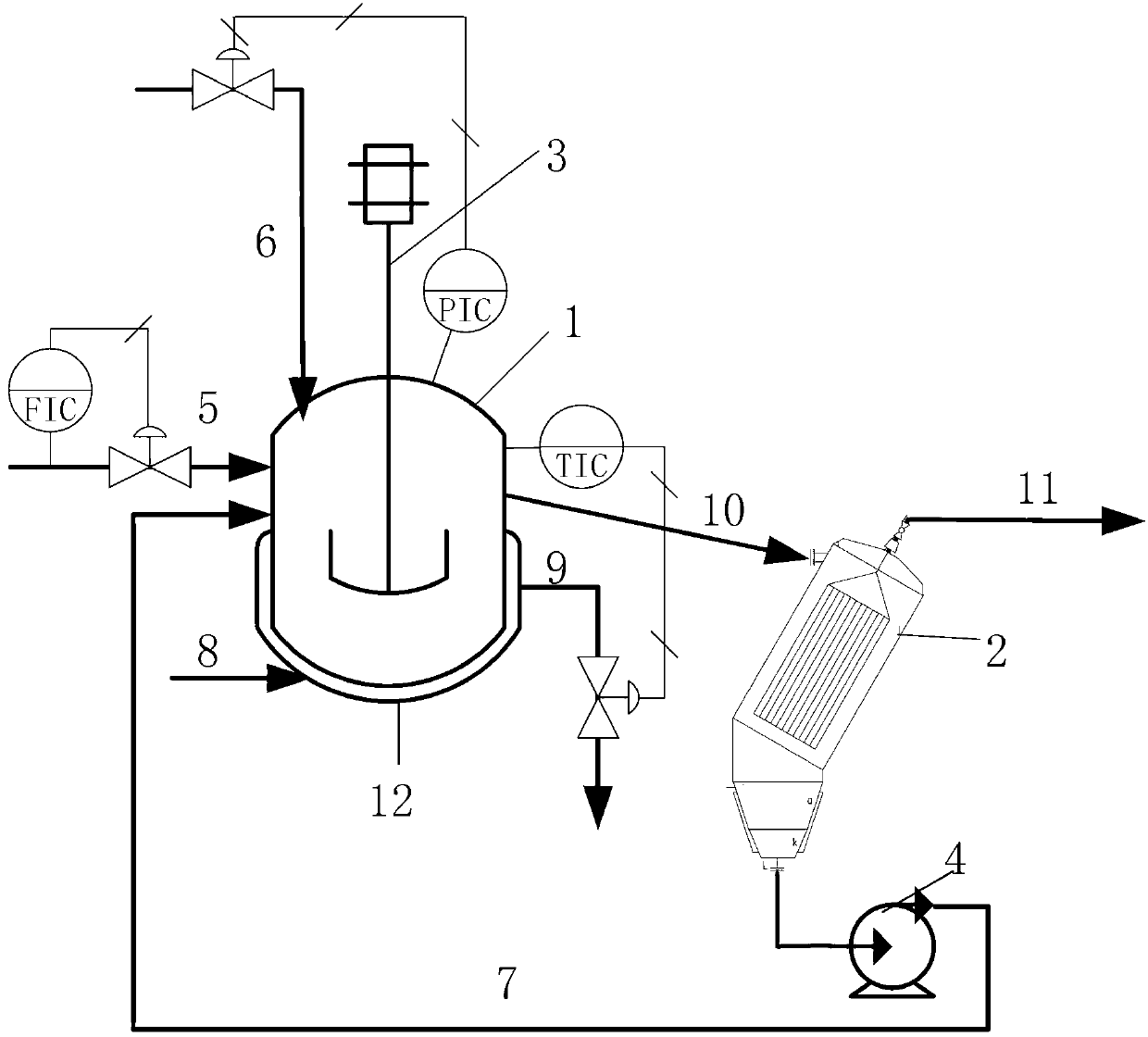
InactiveCN102675127ASolve filtering problemsReduce labor intensityOrganic compound preparationAmino compound preparationAutomatic controlHydrogen
The invention belongs to the field of chemical processing, and particularly discloses a method and a device for preparing 3,4-dichloroaniline without solvent. The method includes using 3,4-dichloronitrobenzene as a raw material, adding an assistant and a novel catalyst into the 3,4-dichloronitrobenzene, feeding hydrogen into a hydrogenation kettle to react continuously under certain pressure and at certain temperature, and collecting reacted hydrogenated liquid into a hydrogenated liquid tank via a filter; and blowing a filtered catalyst into the hydrogenation kettle to be reused via the filter. The method and the device have the advantages that a process is simple in operation, production cost is low, automatic control degree is high, yield of product is also high, quality is good, side reaction is little, reaction conditions are moderate, security is guaranteed, labor intensity of workers is low, and the method and the device are suitable for wide popularization and application.
Owner:山东沾化天九化工有限公司
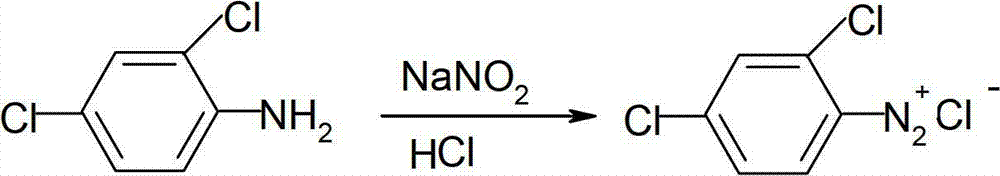
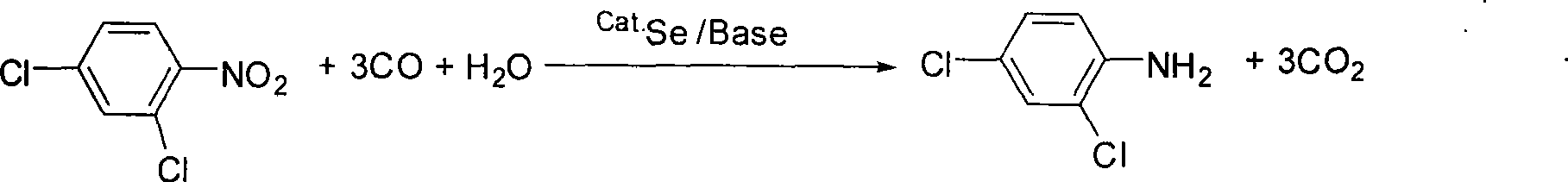
Method for synthesizing 2, 4-dichloroaniline
InactiveCN101445459AChlorine has no effectGood reduction selectivityOrganic compound preparationAmino compound preparationBenzeneOrganic solvent
The invention relates to a method for synthesizing 2, 4-dichloroaniline. The adopted technical scheme is as follows: in the presence of carbon monoxide and water, 2, 4-dinitrobenzene serves as the raw material, selenium serves as a catalyst, and alkali serves as a cocatalyst, or no cocatalyst is added; a nitro selective reduction reaction is conducted in organic solvent under high pressure; the molar ratio of 2, 4-dichloronitrobenzene and water is 1:1 to 100; the mole amount of selenium is 0.2 to 10 percent of 2, 4-dinitrobenzene; the mole amount of alkali is 0 to 200 percent of 2, 4-dinitrobenzene; the reaction temperature is 80 to 200 DEG C; the reaction pressure is 1 to 6Mpa, the reaction time is 2 to 10 hours; and after reaction, the organic solvent is cooled to room temperature; reaction waste gas is exhausted; oxygen or air is introduced into the organic solvent and agitated for 1 to 2 hours; and the organic solvent is filtered and distilled. The method uses selenium as the catalyst, and the reduction selectivity is up to more than 99 percent, which is close to the reduction selectivity of a quantitative reaction.
Owner:LIAONING UNIVERSITY
Method for synthetizing 3,5-dichloroaniline
InactiveCN103508901AReduce pollutionWith sustainable developmentOrganic compound preparationAmino compound preparationO-nitrochlorobenzeneChlorobenzene
The invention discloses a method for synthetizing 3,5-dichloroaniline by using industrial production residues, namely meta-position oil, of p-nitrchlorobenzene and ortho-nitrochlorobenzene by two steps of chlorination and reductive dechlorination. The method adopts the following technical scheme: adding an organic solvent to nitrochlorobenzene meta-position oil as a raw material, directly producing a quintozene mixture by one-step chlorination under the effect of a catalyst, reacting for 0.5-10 hours under the condition of 10-180 DEG C, and then cooling to 30 DEG C; filtering out quintozene, and putting into an autoclave after washing into a neutral state by hot water; carrying out dechlorination reduction reaction under the effects of an organic solvent and the catalyst, wherein the reaction temperature is 60-200 DEG C, the reaction pressure is 1-20 MPa, and the reaction time is 1-20 hours; cooling to room temperature after the reaction is ended; leading in oxygen or air to stir after blowing off; filtering and distilling under reduced pressure to remove the solvent, so as to obtain the product 3,5-dichloroaniline, wherein the yield is 80-90%. Waste materials are changed into precious materials by a synthetic route adopted by the technology disclosed by the invention; the target of zero emission is achieved; the method has the advantages of sustainable development, energy conservation and consumption reduction, and small environmental pollution.
Owner:CHINA PETROLEUM & CHEM CORP +1
Preparation method for catalyst used for preparation of chlorinated arylamines through catalytic hydrogenation
ActiveCN107970967APrevent restoreEvenly distributedPhysical/chemical process catalystsOrganic compound preparationBenzeneAmmonium compounds
The invention relates to a preparation method for a catalyst used for preparation of chlorinated arylamines through catalytic hydrogenation, and specifically to a preparation method for a supported noble metal complex catalyst and an application of the supported noble metal complex catalyst in preparation of the chlorinated arylamines like o-chloroaniline, 3,4-dichloroaniline and 2,5-dichloroaniline through catalytic hydrogenation. The invention provides a preparation method for a carbon-supported catalyst (Pt-N / C or Pd-N / C for short, wherein N represents one or more selected from the group consisting of inorganic ammonium compounds) which is obtained through an action of noble metal and an inorganic ammonium compound; and the catalyst is used for preparation of the chlorinated arylaminesthrough catalytic hydrogenation of chloronitrobenzene. The preparation processes for the catalyst and the chlorinated arylamines have the following main advantages: 1, little difference is generated between preparation processes of the catalyst and ordinary noble metal carbon-supported catalysts, and the preparation processes are simple; 2, continuous addition of an auxiliary agent is not needed in the process of preparation of the chlorinated arylamines through catalytic hydrogenation of chloronitrobenzene by utilizing the catalyst; 3, the catalyst has stable activity and low dechlorination amount in the process of hydrogenation; and 4, no solvent is used in the process of hydrogenation, and production capacity is improved.
Owner:JIANGSU RUIXIANG CHEM +1
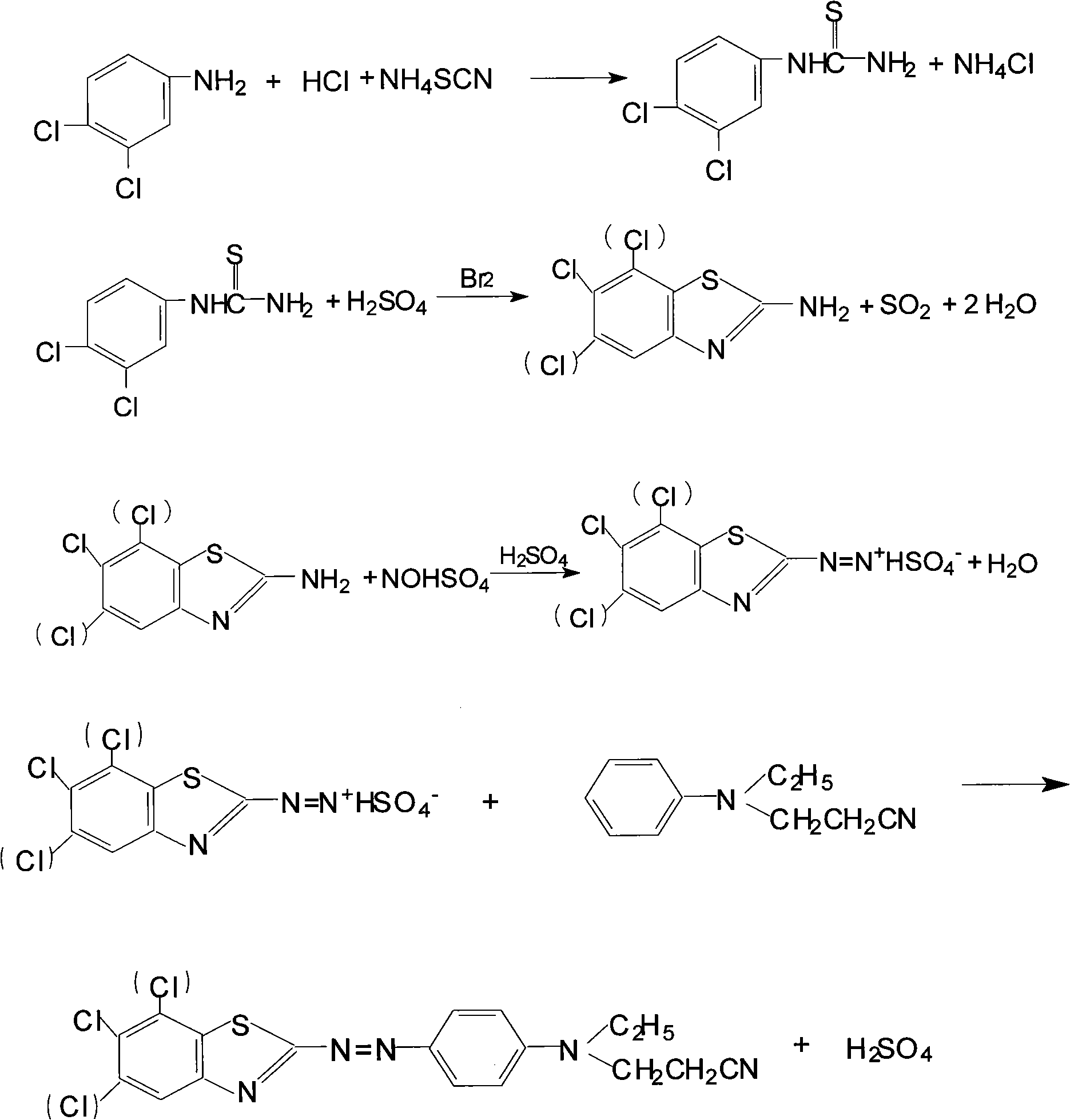
Preparation method of disperse scarlet
The invention relates to a preparation method of disperse scarlet, which is characterized by comprising the steps such as preparation of thiourea, closing ring, diazotization, coupling and the like, wherein the thiourea is prepared by adopting 3, 4-dichloroaniline, hydrochloric acid and ammonium thiocyanate for reaction; a closed ring is prepared by adding sulfuric acid, the thiourea and bromine in a reaction kettle; diazotization means that secondary heavy nitrogen is adopted for reaction to prepare secondary heavy nitrogen material; coupling means that emulsifying agent OP-10, N-ethyl-N-cyanoethyl phenylamine, sulfamic acid, defoaming agent tributyl phosphate and the secondary heavy nitrogen material are adopted for reaction until the coupling reaction finish that the N-ethyl-N-cyanoethyl phenylamine is excessive; and finally the finished product of the disperse scarlet can be obtained by press filtering, washing and drying. The preparation method is more reasonable in technique, and the mother solution can be recovered, so that the product yield is effectively improved, the production cost is reduced, and the discharge of the waste water (COD) is decreased.
Owner:JIANGSU YUANZHENG CHEM
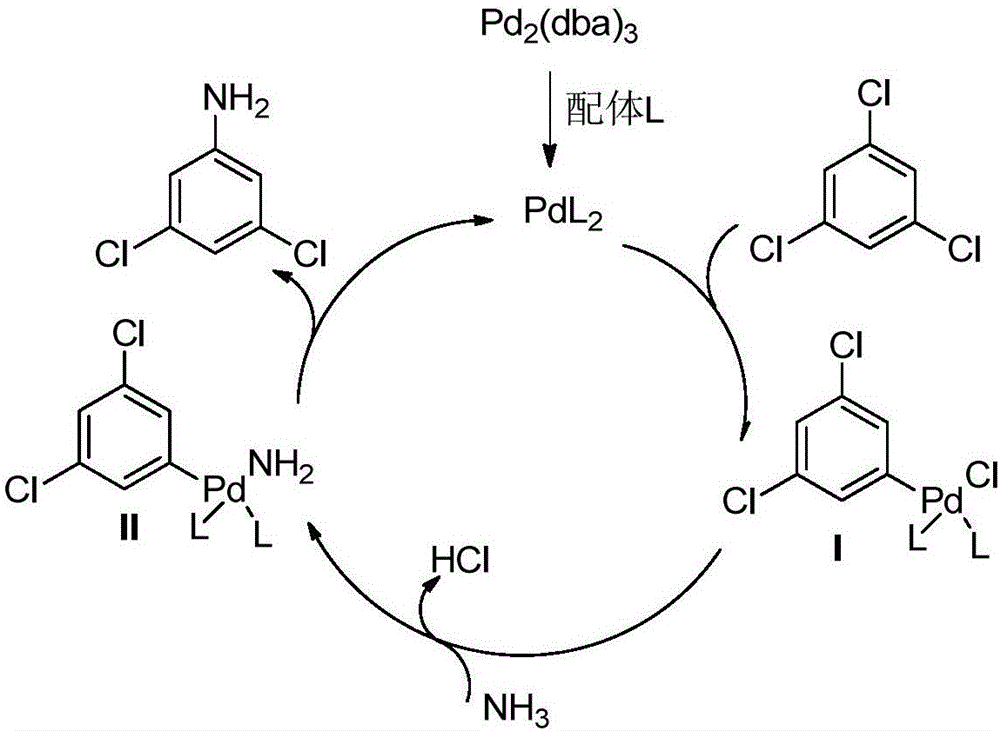
Synthesis method of 3,5-dichloroaniline
ActiveCN106748801ANothing producedTo avoidAmino compound purification/separationOrganic compound preparationOrganic solventSynthesis methods
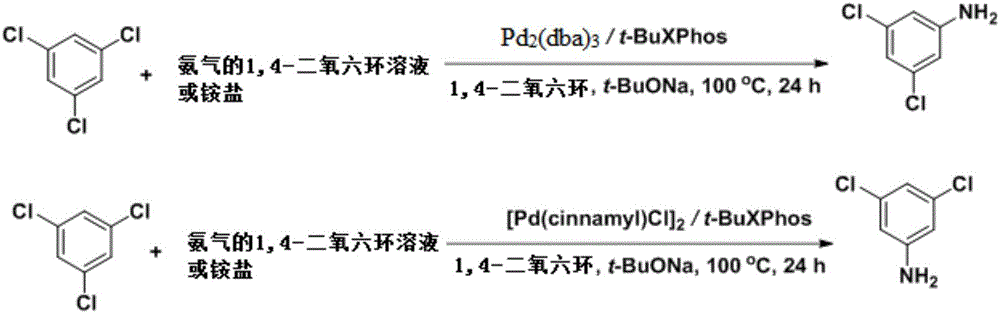
The invention discloses a synthesis method of 3,5-dichloroaniline. 1,3,5-trichlorobenzene serves as a raw material, alkali or an ammonia solution or ammonium salt is added into an organic solvent to serve as an ammonia source, a coupling reaction is generated under the effect of a transition metal catalyst and ligand, and the product 3,5-dichloroaniline is obtained. According to the method, the raw materials are simple and easy to obtain, the cost is low, synthesis steps are simple, the product 3,5-dichloroaniline can be obtained through direct coupling of 1,3,5-trichlorobenzene and the ammonia source only through a one-step reaction, fewer byproducts are generated, fewer three wastes are generated, environmental friendliness is achieved, upgrading of the product process is achieved, and great significance is achieved for reducing the synthesis cost of 3,5-dichloroaniline and derivatives thereof.
Owner:EAST CHINA NORMAL UNIV
Preparation method for synthesizing 2,4-dichloroaniline from 2,4-dichloronitrobenzene
InactiveCN101798269ANo pollutionEasy to operateOrganic compound preparationAmino compound preparationCarbon numberHydrogen
The invention provides a preparation method for synthesizing 2,4-dichloroaniline from 2,4-dichloronitrobenzene. The preparation method comprises the following steps: feeding 2,4-dichloronitrobenzene, solvent and catalysts into a reaction kettle; feeding hydrogen into the reaction kettle for 6 to 16 hours at 20 to 100 DEG C at 3 to 30MPa while stirring, wherein the pressure in the reaction kettle is controlled within 4 to 25MPa; stopping feeding the hydrogen, reducing the temperature, and filtering the catalysts out at the normal pressure when the pressure in the reaction kettle starts to increase slowly; then, distilling 90% of the total solvent at the normal pressure; detecting the amino value of the product, i.e., 2,4-dichloroaniline, and indicating that the finished product is qualified if the amino value thereof is higher than or equal to 99%, wherein the weight percentage of the liquid material, i.e., 2,4-dichloronitrobenzene, is higher than or equal to 99%; the solvent is particularly alcohol and methylbenzene the carbon number of which are smaller than or equal to 4; the catalysts are particularly palladium-carbon series catalysts, Raney nickel and inhibitory amino dechlorination catalysts; and the hydrogen is a commercial product with the content of hydrogen being higher than or equal to 99.5%. The invention has the characteristics of simple technological operation, simple treatment after reduction destination, short production period, three-waste emission avoidance, environmental pollution prevention, high device utilization rate, high product yield, good product quality and the like.
Owner:荆州市恒诚精细化工有限公司
Prepn process of 2,5-dichloro-p-phenylenediamine
InactiveCN1974540AMild reaction conditionsReduce manufacturing costOrganic compound preparationAmino compound preparationHydrazine compoundNitration
The process of preparing 2, 5-dichloro-p-phenylene diamine adopts 2, 5-dichloroaniline as main material, and includes four reaction steps of acylation, nitration, hydrolysis and reduction. The present invention has improved acylation reaction performed in halogenated hydrocarbon as solvent, and improved catalytic reduction of hydrazine hydrate with the catalyst ferric chloride and active carbon performed in alcohol as solvent. The improved process has no exhausted waste acid effluent from acylation reaction and waste iron slime from reduction, high product yield, mild reaction condition, high product purity and low production cost, and is suitable for industrial production.
Owner:上海染料研究所有限公司
Refining method of 3,4-dichlorobenzene isocyanate
ActiveCN103739520AImprove qualityImprove conversion rateOrganic compound preparationIsocyanic acid derivatives purification/separationTolueneReaction speed
The invention provides a refining method of 3,4-dichlorobenzene isocyanate. The process method comprises the following steps: firstly, preparing a 3,4-dichloroaniline methylbenzene solution, and then carrying out phosgene esterification reaction, and finally rectifying. According to the refining method, through continuous rectification, a product contains 99.5% of the 3,4 dichlorobenzene isocyanate, which is 1% higher than purity of a product achieved by common rectification; meanwhile, quality of the finished product is improved; content of methylbenzene after the continuous rectification is 99.5%; besides, the generation of side reactions is reduced when a phosgene reaction is performed, and the conversion rate of the methylbenzene is increased by 3%, therefore, when the 3,4 dichlorobenzene isocyanate reacts with other substances, generation of many byproducts is reduced, the reaction speed is improved, and the yield of the product is increased.
Owner:ANHUI GUANGXIN AGROCHEM
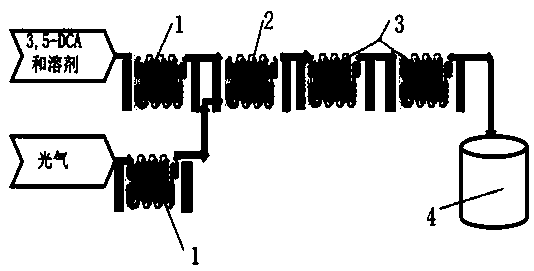
Method for preparing 3,5-dichlorophenyl isocyanate by microchannel reactor
InactiveCN109180530AShort reaction timeImprove reaction efficiencyIsocyanic acid derivatives preparationOrganic compound preparationContinuous flowNitrogen gas
The invention discloses a method for preparing 3,5-dichlorophenyl isocyanate by a microchannel continuous flow reactor. With 3,5-dichloroaniline (3,5-DCA) and gaseous phosgene as raw materials, the 3,5-DCA and a solvent are firstly mixed to form a homogeneous solution in a Corning microchannel reactor; the homogeneous solution and phosgene are preheated respectively, introduced into a first microchannel reactor, mixed and subjected to a reaction; the obtained mixture is introduced into two microchannel reactors in series subsequently to obtain a 3,5-dichlorophenyl isocyanate solution; and thenexcess phosgene is removed by nitrogen gas, and 3,5-dichlorophenyl isocyanate is obtained by reduced pressure rectification. The preparation method shortens the reaction time from a few hours of a traditional method to a few minutes, allows the reaction time generally not to exceed 30 min, the reaction efficiency is significantly improved, the selectivity of the product is significantly improved,a by-product urea is obviously reduced, the three wastes are reduced, the reaction yield is increased, the yield reaches 95-98%, the purity of the product is more than 99%, and the production cost islow.
Owner:JIANGXI HEYI CHEM
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com