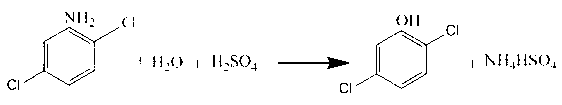Synthesis method of 2, 5-dichlorophenol
A synthesis method and technology of dichlorophenol, applied in directions such as organic chemistry, can solve problems such as unfavorable clean production, large equipment investment, long reaction time, etc., and achieve the effects of increasing effective output, reducing raw material consumption, and reducing three wastes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Add 636g of water to a 1000ml four-neck flask, then take 50g of concentrated sulfuric acid with a mass fraction of 98%, and add it to the above flask under stirring to form a 3.68% dilute sulfuric acid solution. After the mixing is complete, add 81.8g 2,5-dichloroaniline with a mass fraction of 98%, then heated to 92°C, and kept warm until the material was completely dissolved. The material was then transferred to a 2L zirconium autoclave. Nitrogen was passed to 0.8Mpa to replace the gas inside, and then the autoclave was sealed for heating. Raise the temperature to 160°C, keep it warm for 2 hours, then lower the temperature, wait until the temperature drops to 60°C, open the air, then stand still, layer and separate liquids to obtain 629.8.1g of water phase, 0.12% of phenol content, 86g of oil phase, 90.6% of phenol content , the oil phase was decompressed and rectified under a vacuum of 0.099Mpa, and a total of 77.3g of fractions above 70°C were collected. The conten...
Embodiment 2
[0023] Add 950g of water to a 1000ml four-neck flask, then take 100g of concentrated sulfuric acid with a mass fraction of 98%, and add it to the above flask under stirring to form a 9.33% dilute sulfuric acid solution. After the mixing is complete, add 81.8g 2,5-dichloroaniline with a mass fraction of 98%, then heated to 90°C, and kept warm until the material was completely dissolved. The material was then transferred to a 2L zirconium autoclave. Nitrogen was passed to 5.0Mpa to replace the gas inside, and then the autoclave was sealed for heating. Raise the temperature to 280°C, keep it warm for 3 hours, then lower the temperature, wait until the temperature drops to 50°C, open the air, then stand still, separate layers and separate liquids to obtain 1044.1g of water phase with 0.13% phenol content, 86.1g of oil phase with 90.3% phenol content , the oil phase was decompressed and rectified under a vacuum of 0.099Mpa, and a total of 77g of fractions above 70°C were collected...
Embodiment 3
[0025] Add 861g of water to a 1000ml four-neck flask, then take 108.9g of concentrated sulfuric acid with a mass fraction of 98%, and add it to the above flask under stirring to form a 11.00% dilute sulfuric acid solution. After mixing, add 81.8 g of 2,5-dichloroaniline with a mass fraction of 98%, then heated to 90°C, and kept warm until the material was completely dissolved. The material was then transferred to a 2L zirconium autoclave. Nitrogen was passed to 3.0Mpa to replace the gas inside, and then the autoclave was sealed for heating. Raise the temperature to 180°C, keep it warm for 5 hours, then lower the temperature, wait until the temperature drops to 70°C, open the air, then stand still, layer and separate liquids to obtain 964.7g of water phase, phenol content 0.18%, 85.6g of oil phase, phenol content of oil phase 91.3%, yield 96.4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com