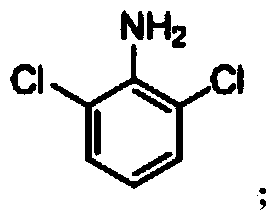
Synthetic method of 2, 6-dichloroaniline
A technology of dichloroaniline and a synthesis method, which is applied in chemical instruments and methods, preparation of amino compounds, preparation of organic compounds, etc., can solve the problem that the cooling effect of decomposition and chlorination reaction equipment is also very high, and 1,3-diphenylene High quality requirements of urea, large equipment and environmental impact, etc., to achieve the effect of less corrosion, simple post-treatment, and small equipment investment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
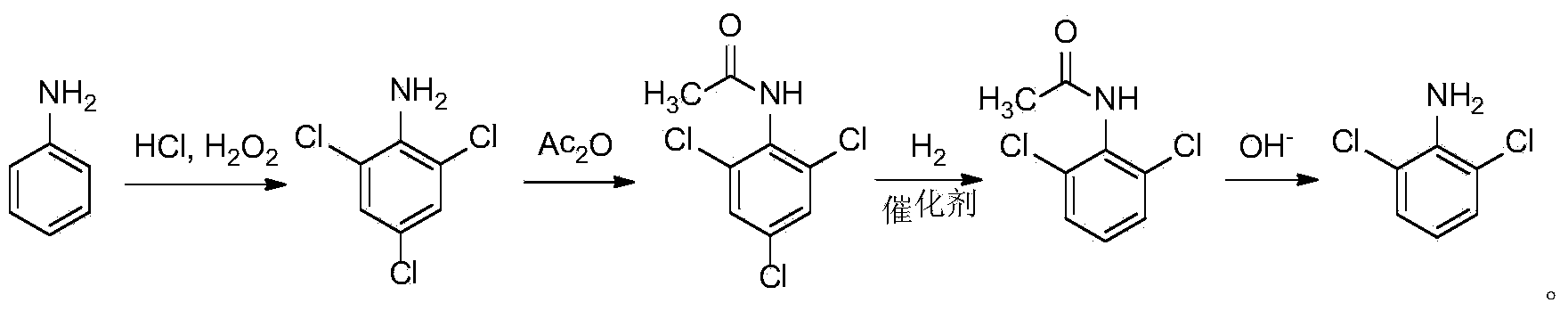
[0042] Embodiment 1, a kind of synthetic method of 2,6-dichloroaniline, take aniline as raw material, carry out the following steps successively:
[0043] (1) Put 13.8g (0.15mol) of aniline, 350mL of 35% concentrated hydrochloric acid (4.0mol) and 350mL of water into a 1L three-necked flask, add 56.3g (0.45mol) of 30wt% hydrogen peroxide dropwise under mechanical stirring, and control the temperature at 60 °C, react for 2h. After the reaction was completed, the reaction solution was cooled in an ice bath to precipitate a solid, which was filtered to obtain 2,4,6-trichloroaniline hydrochloride. The solid 2,4,6-trichloroaniline hydrochloride was added to 300 mL of water, followed by steam distillation to obtain 27.3 g (0.14 mol) of solid 2,4,6-trichloroaniline, with a yield of 92.5%.
[0044] (2) Add the solid 2,4,6-trichloroaniline obtained in step 1) into a 500mL three-necked flask, add 23.1g (0.23mol) of acetic anhydride dropwise under ice bath conditions and mechanical stir...
Embodiment 2
[0047] Embodiment 2, a kind of synthetic method of 2,6-dichloroaniline, take aniline as raw material, carry out the following steps successively:
[0048] (1) Put 13.8g (0.15mol) of aniline, 350mL of 35% concentrated hydrochloric acid and 350mL of water into a 1L three-necked flask, add 56.3g (0.45mol) of 30wt% hydrogen peroxide dropwise under mechanical stirring, control the temperature at 80°C, and react for 1h. After the reaction was completed, the reaction solution was cooled in an ice bath to precipitate a solid, which was filtered to obtain 2,4,6-trichloroaniline hydrochloride. The solid 2,4,6-trichloroaniline hydrochloride was added to 300 mL of water, and steam distillation was performed to obtain 27.0 g (0.14 mol) of solid 2,4,6-trichloroaniline, with a yield of 91.7%.
[0049](2) Add the solid 2,4,6-trichloroaniline obtained in step 1) into a 500mL three-neck flask, add 30.6g (0.30mol) of acetic anhydride dropwise under ice bath conditions and mechanical stirring, an...
Embodiment 3
[0052] Embodiment 3, a kind of synthetic method of 2,6-dichloroaniline, take aniline as raw material, carry out the following steps successively:
[0053] (1) Put 13.8g (0.15mol) of aniline, 350mL of 35% concentrated hydrochloric acid and 350mL of water into a 1L three-neck flask, add 56.3g (0.45mol) of 30wt% hydrogen peroxide dropwise under mechanical stirring, control the temperature at 40°C, and react for 3h. After the reaction was completed, the reaction solution was cooled in an ice bath to precipitate a solid, which was filtered to obtain 2,4,6-trichloroaniline hydrochloride. The solid 2,4,6-trichloroaniline hydrochloride was added to 300 mL of water, and steam distillation was performed to obtain 27.4 g (0.14 mol) of solid 2,4,6-trichloroaniline, with a yield of 93.1%.
[0054] (2) Add the solid 2,4,6-trichloroaniline obtained in step 1) into a 500mL three-necked flask, add 45.9g (0.45mol) of acetic anhydride dropwise under ice bath conditions and mechanical stirring, a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com