Synthesis method of 3,5-dichloroaniline
A synthesis method, technology of dichloroaniline, applied in chemical instruments and methods, preparation of amino compounds, preparation of organic compounds, etc., can solve the problems of high pressure, long reaction time, and difficult separation, and achieve short reaction steps and easy operation Simple, Wide-Source Effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
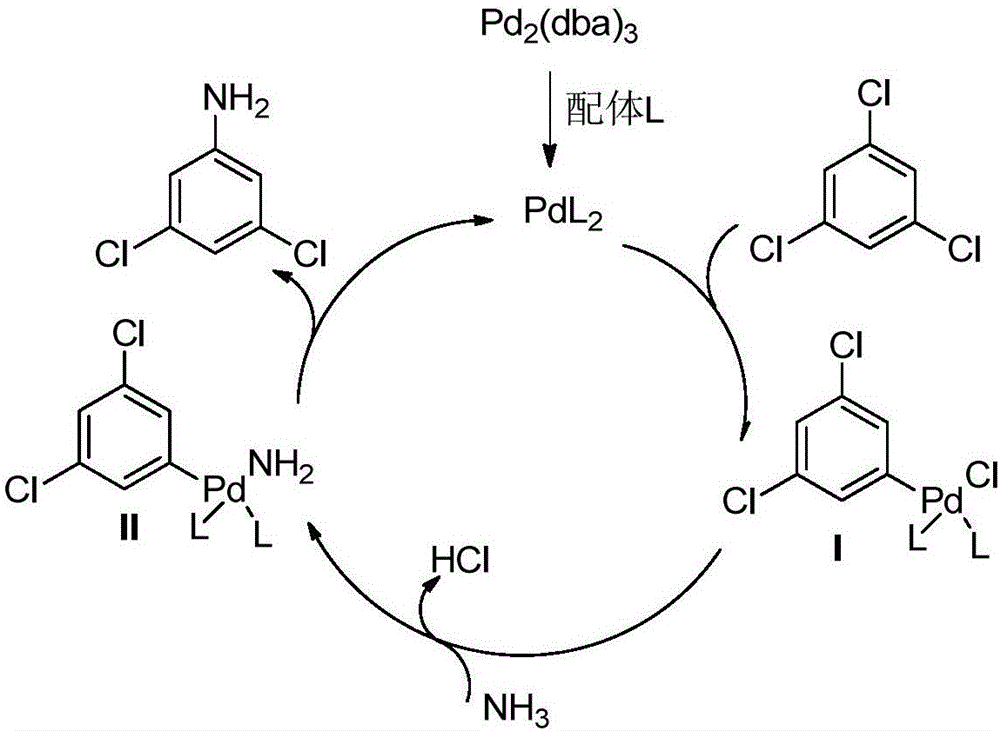
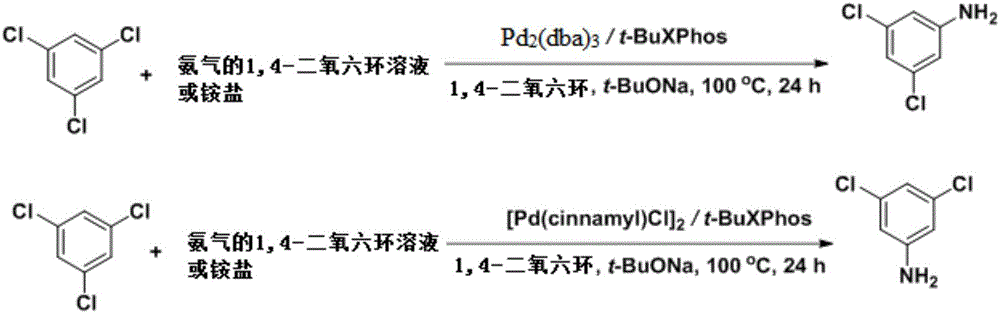
Image
Examples
Embodiment 1
[0036] Add tris(dibenzylideneacetone)dipalladium (0.005mmol) and 2-di-tert-butylphosphino-2',4',6'-triisopropylbiphenyl (0.02mmol) under nitrogen protection in a reaction tube , add 1mL of 1,4-dioxane solvent and stir for 20-60 minutes, the raw material 1,3,5-trichlorobenzene (0.5mmol), sodium tert-butoxide (2.25mmol), ammonium sulfate (0.75mmol) Place in a sealed tube, pump and ventilate under nitrogen protection, then add the coordinated catalyst, then add 5mL of solvent, seal the tube, heat to 100°C and stir for 24h, after the reaction is complete, the raw materials are detected by GCMS. After the reaction, add 5 mL of water to dilute, add 5 mL of tert-butyl methyl ether to extract three times, then add hydrochloric acid to adjust the pH11, extract three times with 5 mL of tert-butyl methyl ether, remove The product 3,5-dichloroaniline (72 mg, 89%) could be obtained by solvent removal.
Embodiment 2
[0038] Add tris(dibenzylideneacetone)dipalladium (0.005mmol) and 2-di-tert-butylphosphino-2',4',6'-triisopropylbiphenyl (0.02mmol) under nitrogen protection in a reaction tube , add 1mL of 1,4-dioxane solvent and stir for 20-60 minutes, the raw material 1,3,5-trichlorobenzene (0.5mmol), sodium tert-butoxide (2.25mmol), ammonium sulfate (0.75mmol) Put it in a sealed tube, pump it for nitrogen protection, then add the coordinated catalyst, then add 2mL of 0.4mol / L ammonia gas 1,4-dioxane solution, and finally add 3mL of solvent, seal it up tube, heated to 100°C and stirred for 24h, after the reaction was completed, the reaction of the raw materials was detected by GCMS. After the reaction, add 5 mL of water to dilute, add 5 mL of tert-butyl methyl ether to extract three times, then add hydrochloric acid to adjust the pH11, extract three times with 5 mL of tert-butyl methyl ether, remove The product 3,5-dichloroaniline (69 mg, 85%) could be obtained by solvent removal.
Embodiment 3
[0040] Add bis-bis[(1,2,3)-1-phenyl-2-propene]dipalladium (0.005mmol) and 2-di-tert-butylphosphino-2',4' under nitrogen protection in a reaction tube, 6'-triisopropylbiphenyl (0.02mmol), add 1mL of 1,4-dioxane solvent and stir for 20-60 minutes, the raw material 1,3,5-trichlorobenzene (0.5mmol), tert-butyl Sodium alkoxide (2.25mmol) and ammonium sulfate (0.75mmol) were placed in a sealed tube, pumped and ventilated under nitrogen protection, then the coordinated catalyst was added, and 5mL of solvent was added, the sealed tube was sealed, heated to 100°C and Stir for 24 hours, after the reaction is completed, it is detected by GCMS that the reaction of the raw materials has been completed. After the reaction, add 5 mL of water to dilute, add 5 mL of tert-butyl methyl ether to extract three times, then add hydrochloric acid to adjust the pH11, extract three times with 5 mL of tert-butyl methyl ether, remove The product 3,5-dichloroaniline (73 mg, 90%) was obtained by solvent r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com