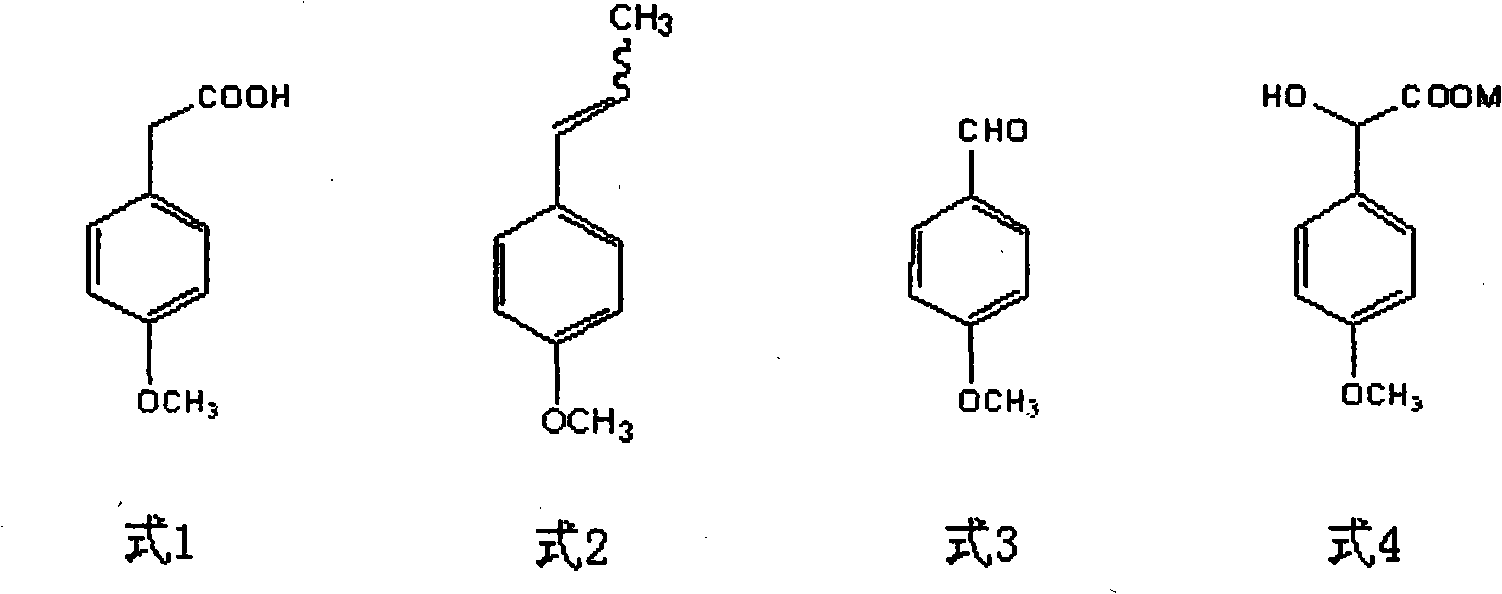
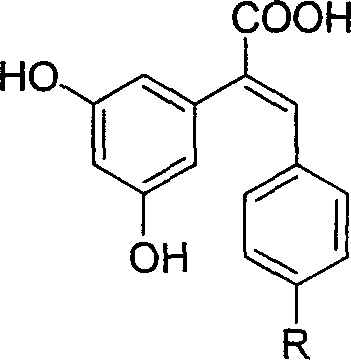
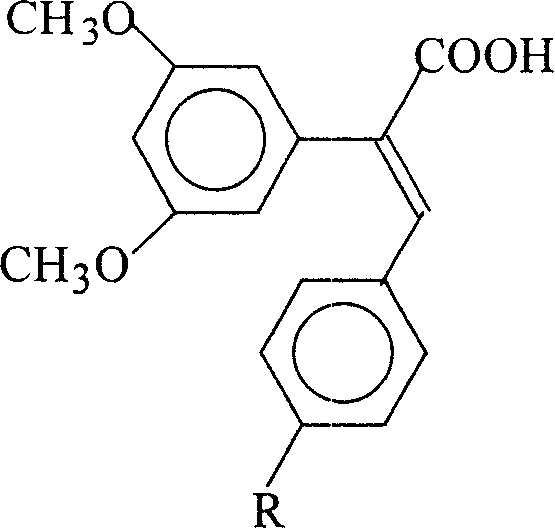
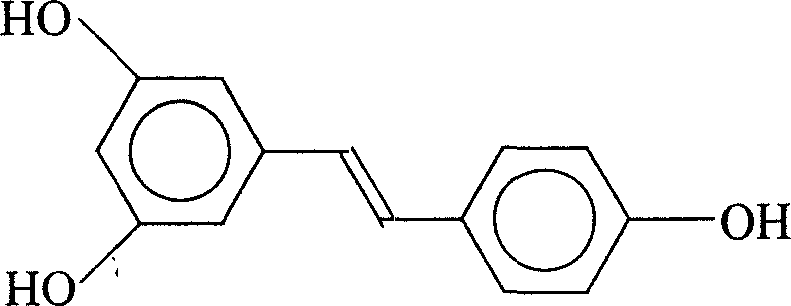
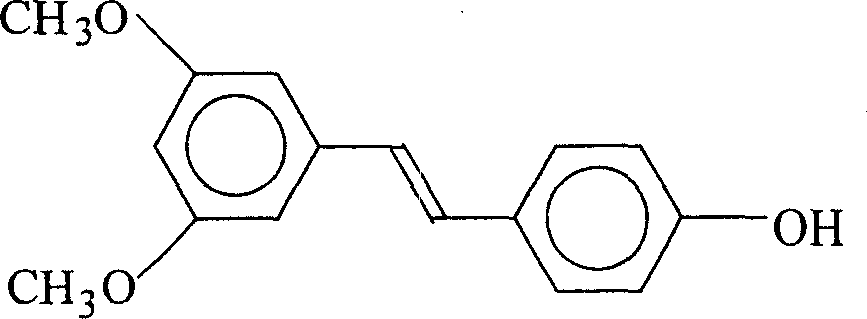
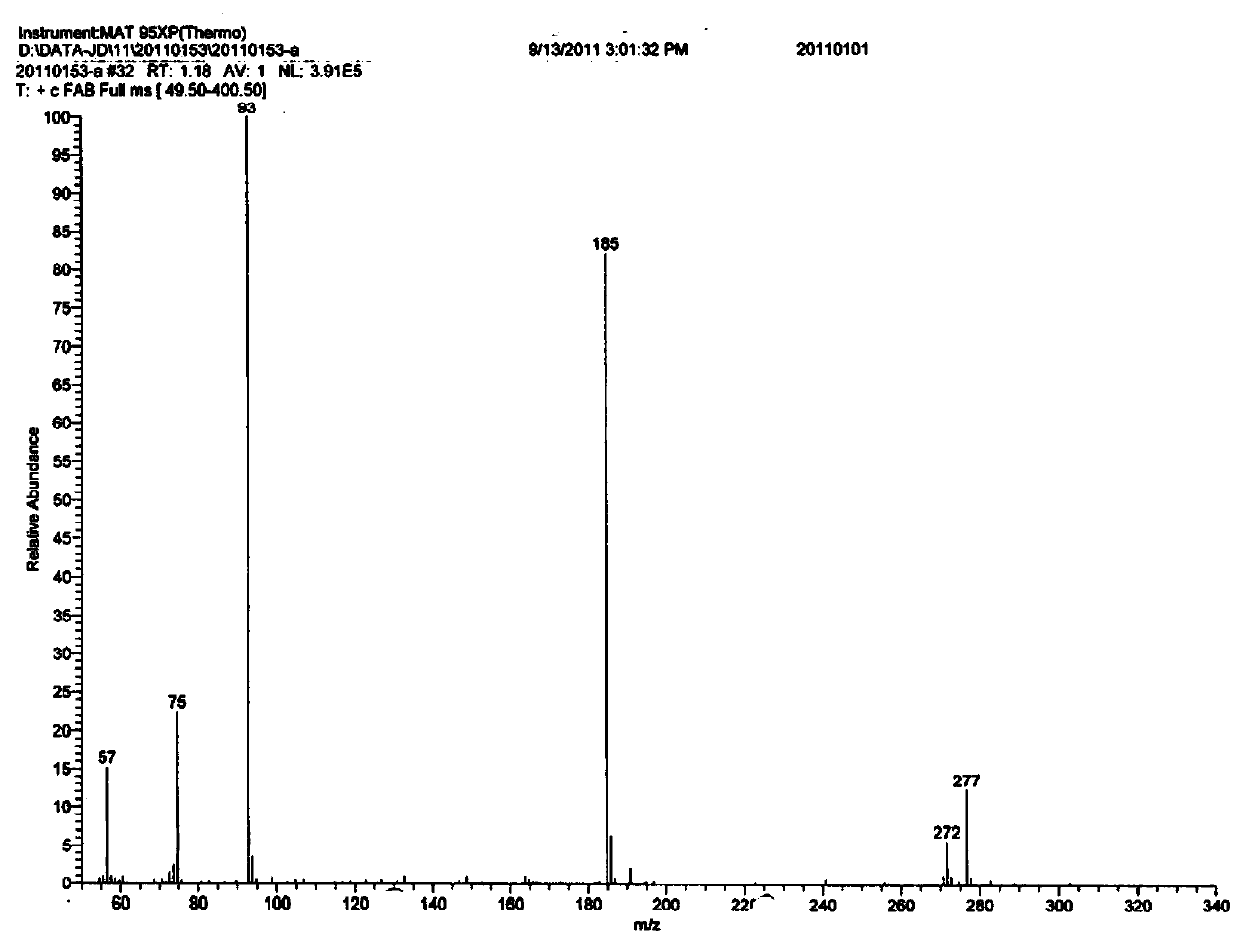
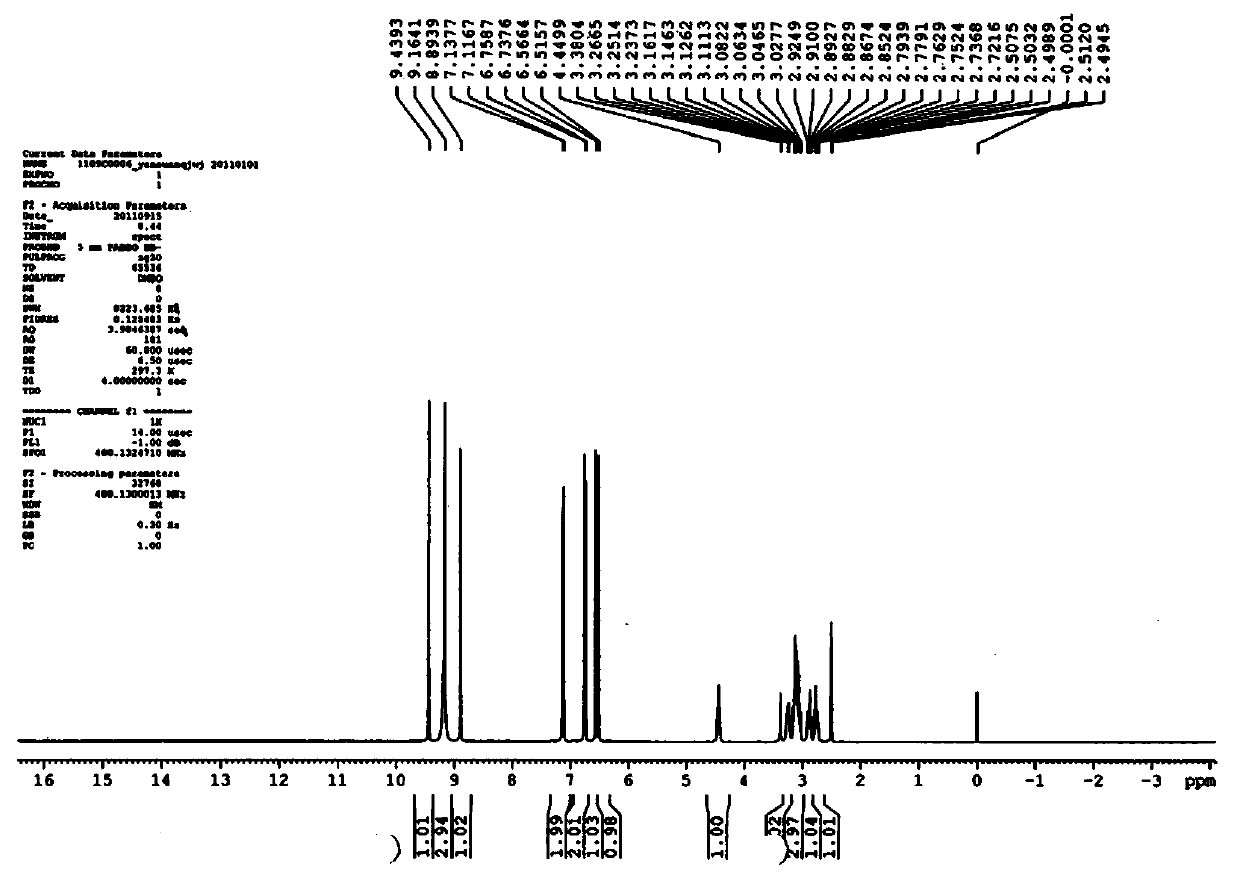
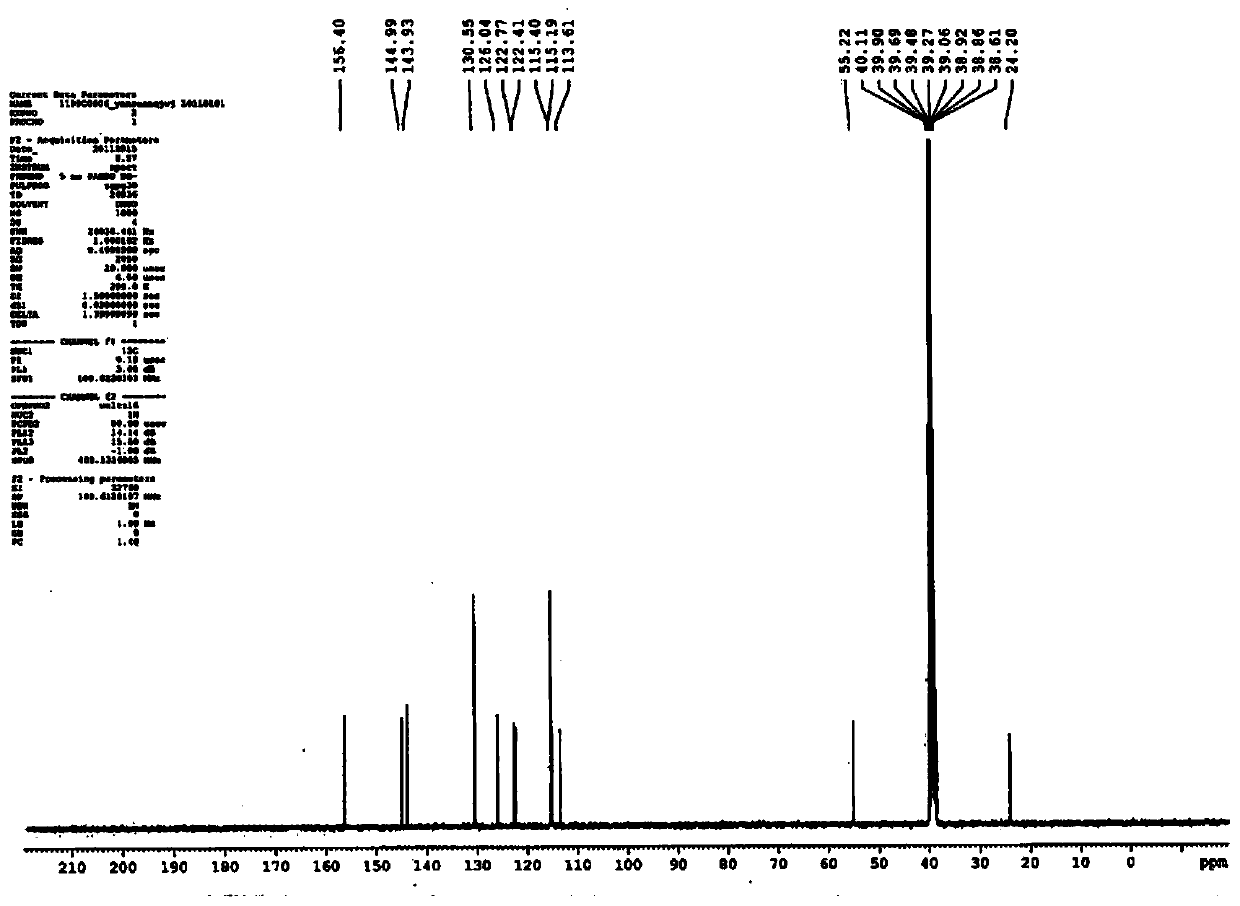
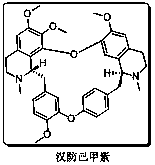
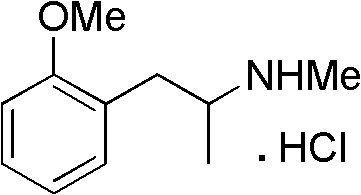
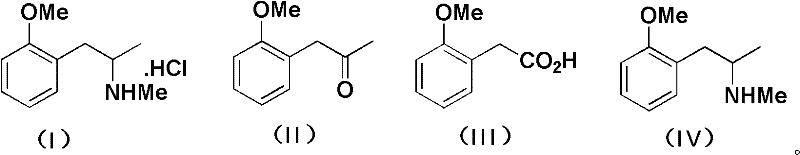
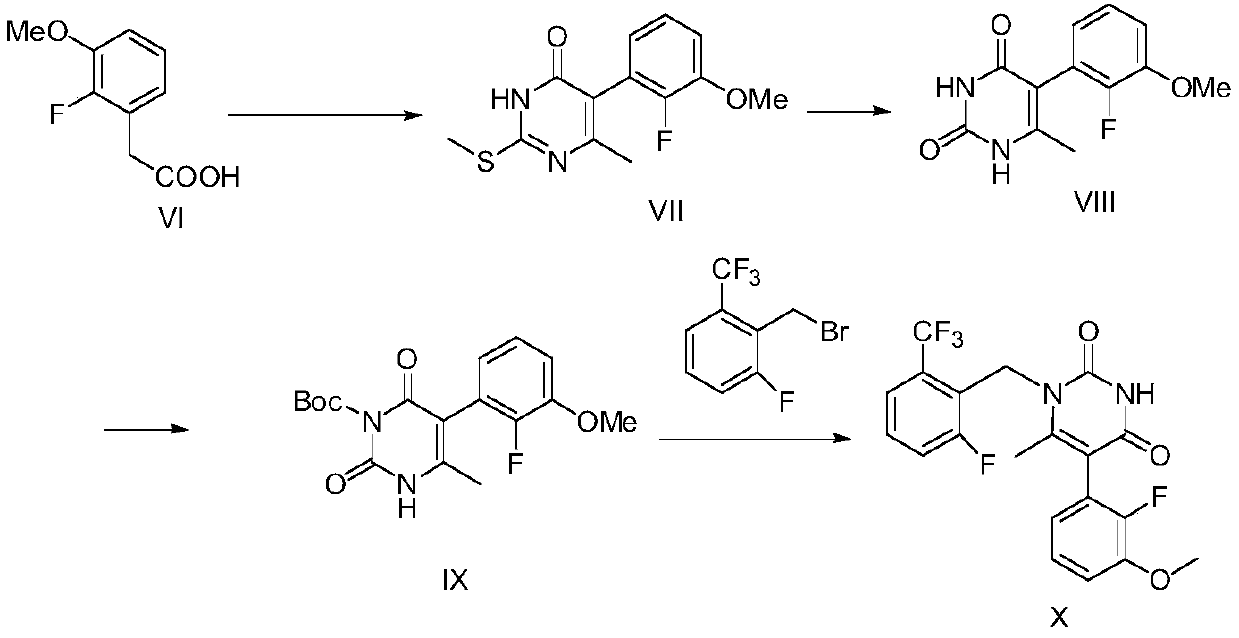
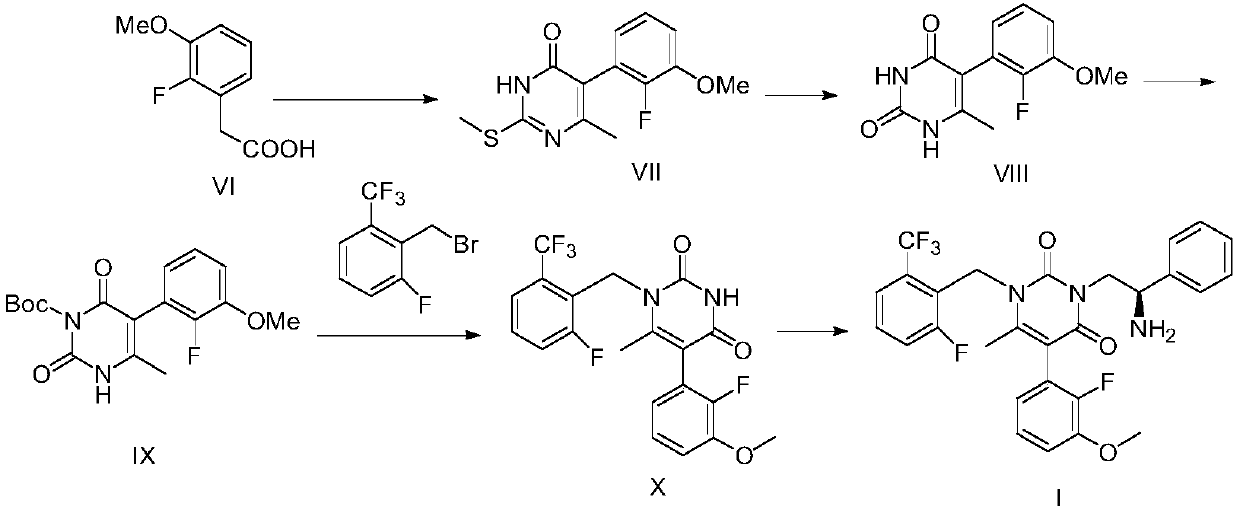
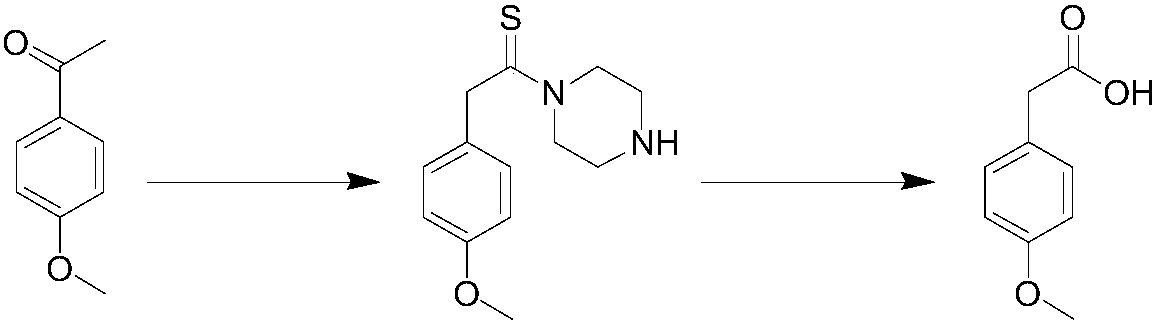
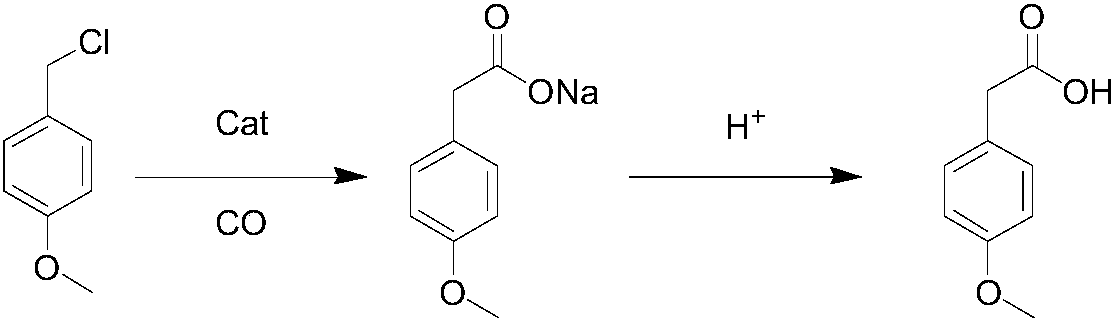
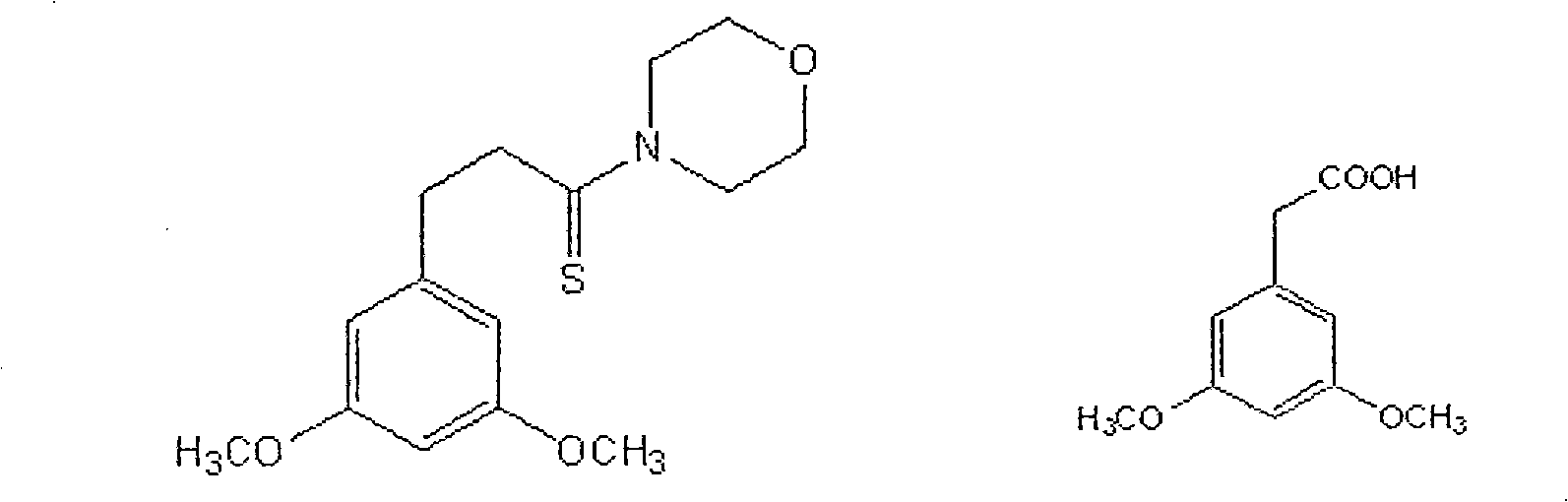
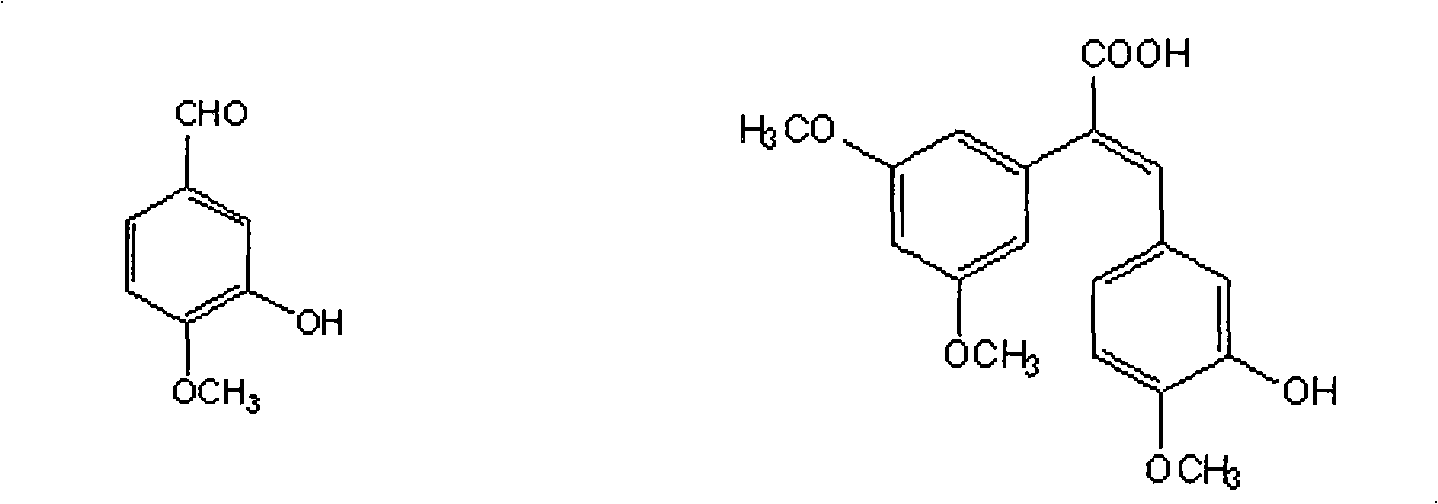
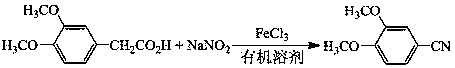Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
94 results about "Methoxy-phenylacetic acid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
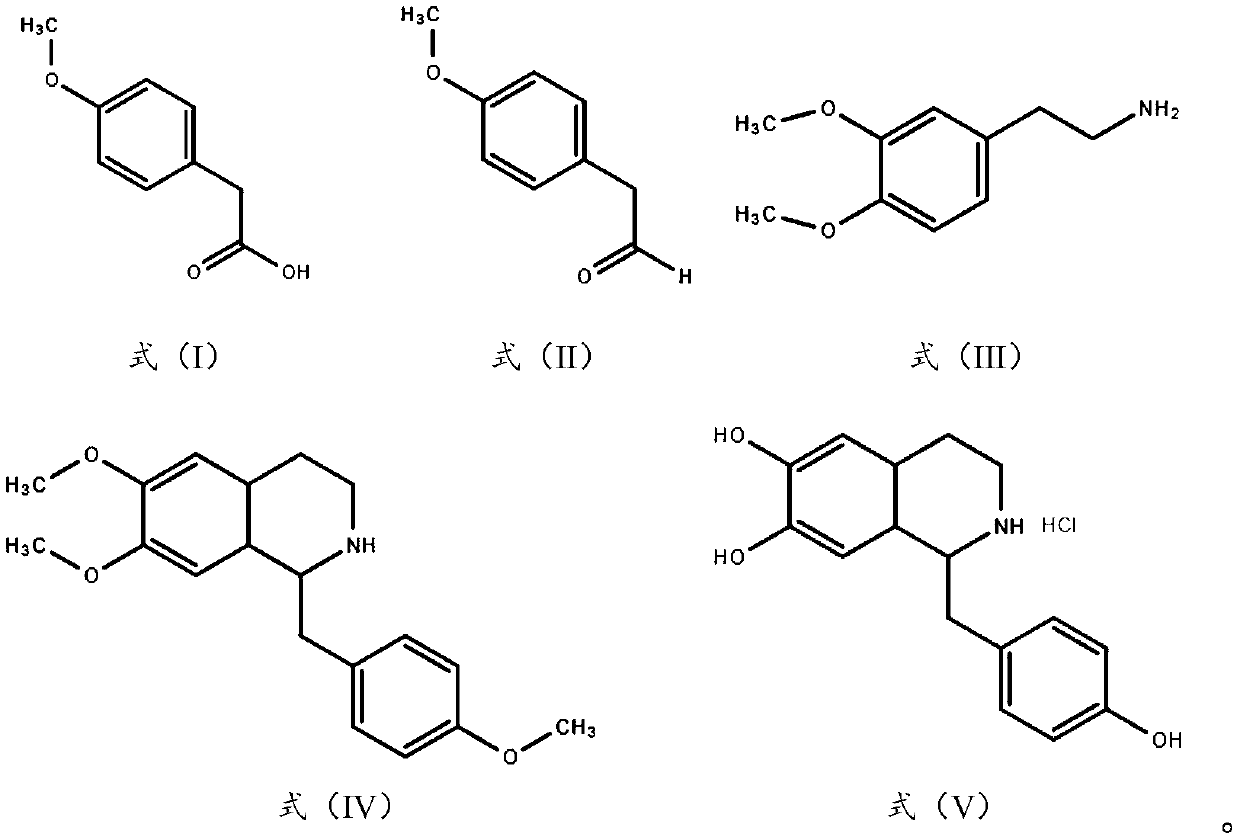
Method for preparing p-methoxypheny-lethyl acid from natural anethole
InactiveCN101298416ANo pollution in the processSimple reaction conditionsOrganic compound preparationCarboxylic preparation by oxidationP-methoxyphenylacetic acidPetrochemical
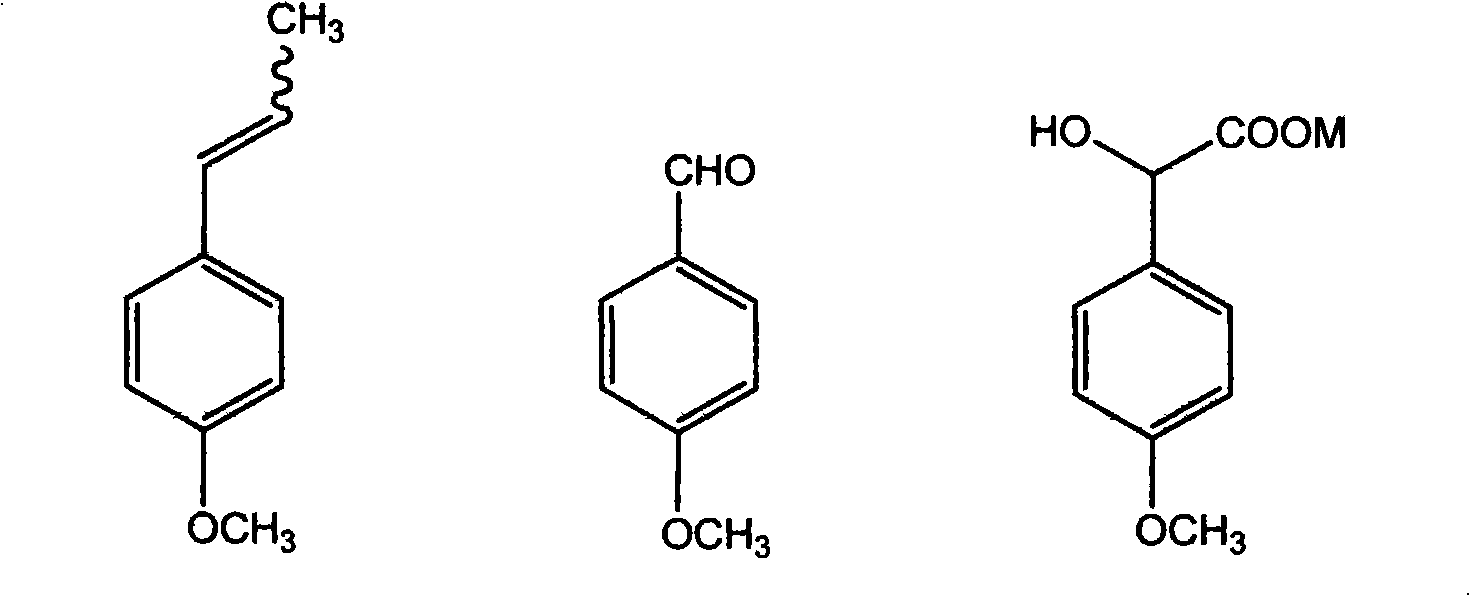
The invention discloses a method for preparing methoxyphenylacetic acid by natural anethole; the natural anethole is taken as a raw material and generated into the anisicaldehyde by oxidation reaction, then anisic mandelic acid (salt) is generated by the insertion reaction of carbine; finally the methoxyphenylacetic acid is obtained by reduction. The method has the advantages of reproducible raw material, simple operation and high yield, etc.; furthermore, the method can prepare the methoxyphenylacetic acid which can replace the source of petrochemical industry.
Owner:GUANGZHOU INST OF GEOCHEMISTRY - CHINESE ACAD OF SCI
Preparation method for 5'-methoxylaudanosine
InactiveCN103880744AMild reaction conditionsReduce energy consumptionOrganic chemistryPotassium borohydrideHydrogen
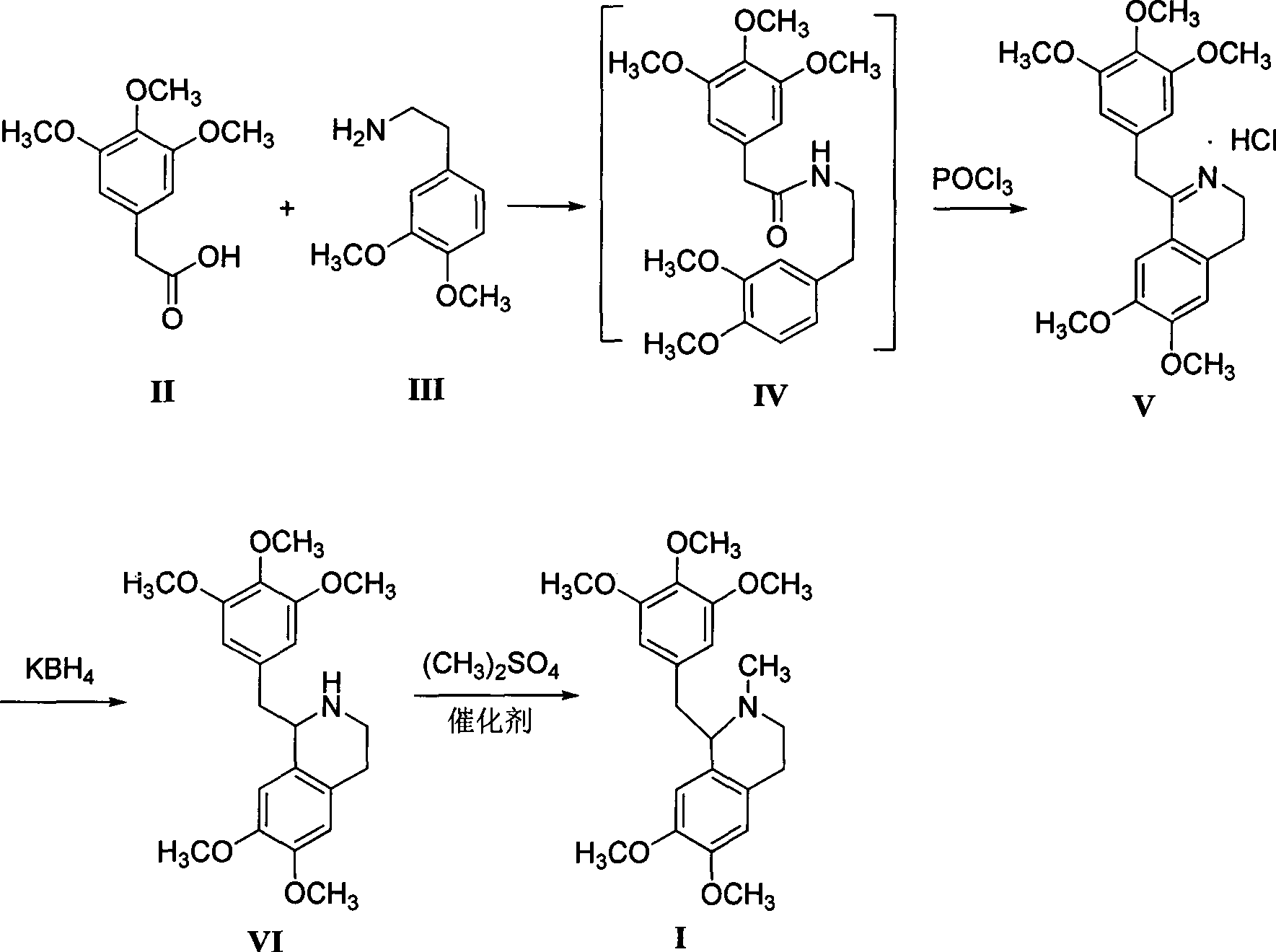
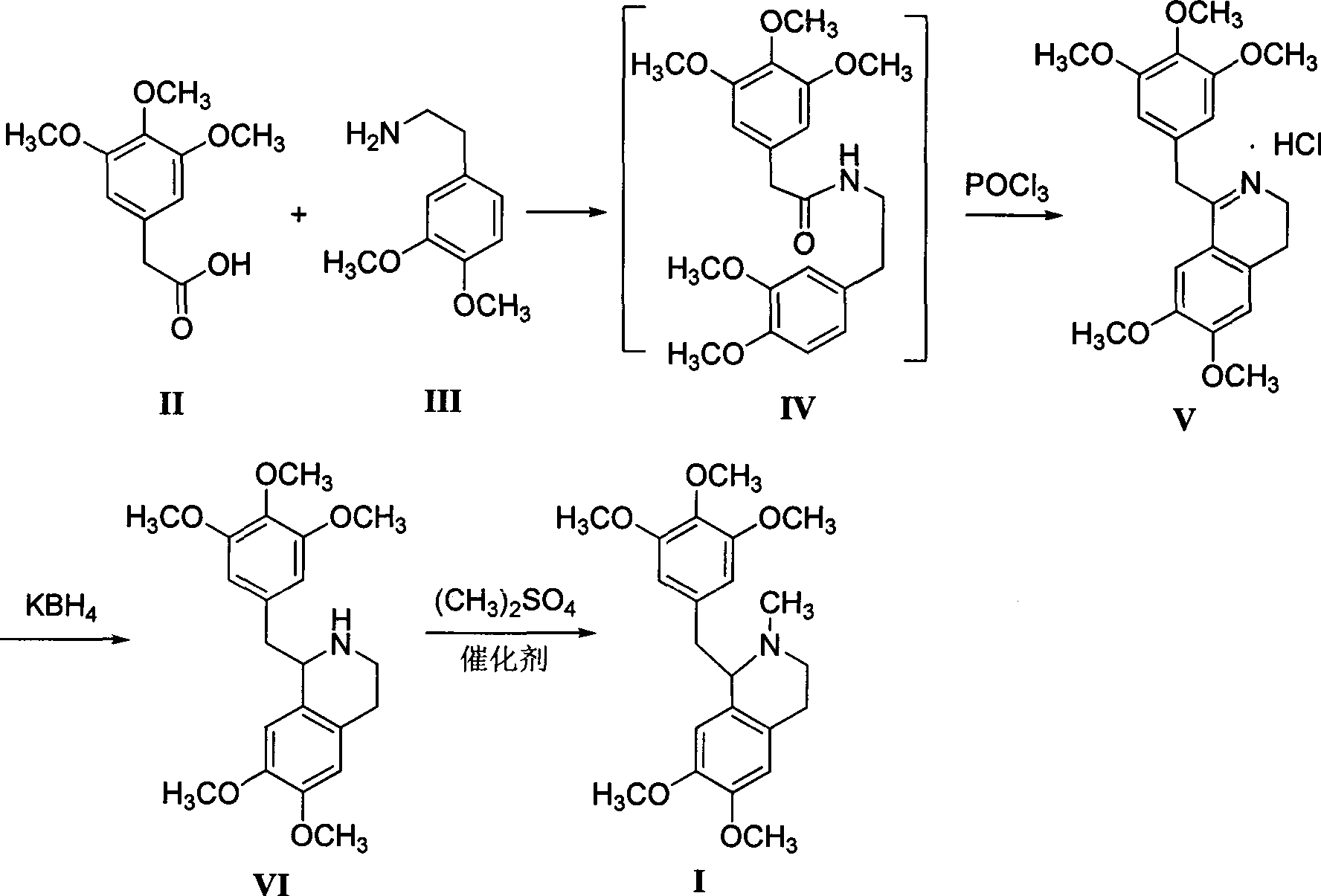
The invention relates to the field of medicine synthesis, and specifically relates to a preparation method for a nondepolarizer mivacurium chloride intermediate 5'-methoxylaudanosine. The preparation method is characterized by taking 3,4,5-trimethoxyphenylacetic acid and 3,4-dimethoxyphenethylamine as raw materials, employing a condensation cyclization one-pot process to prepare 3,4-dihydro-6,7-dimethoxy-1-[2-(3,4,5-trimethoxyphenyl)ethyl]isoquinoline hydrochloride, and then performing potassium borohydride reduction and dimethyl sulphate methylation. The preparation method has the advantages of being mild in reaction conditions, good in product quality, high in yield, low in production cost and the like.
Owner:CHANGZHOU VOCATIONAL INST OF ENG
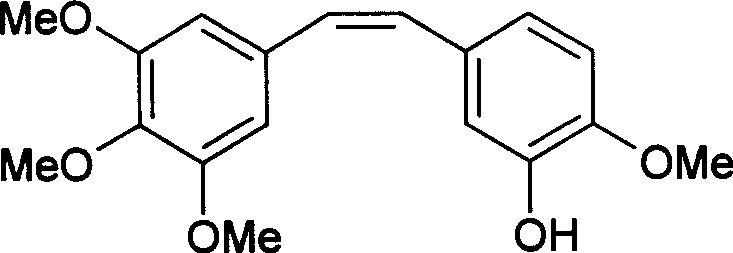
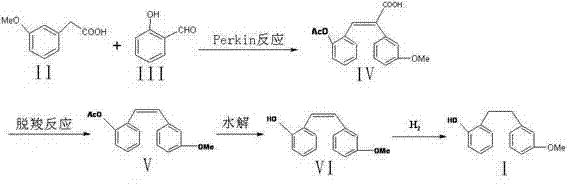
Method of preparing (Z)-3'-hydroxyl-3,4,4',5-tetramethoxy toluylene
InactiveCN101402555AGood sustainable development abilityCost-effectiveOrganic chemistryOrganic compound preparationPetrochemicalMandelic acid
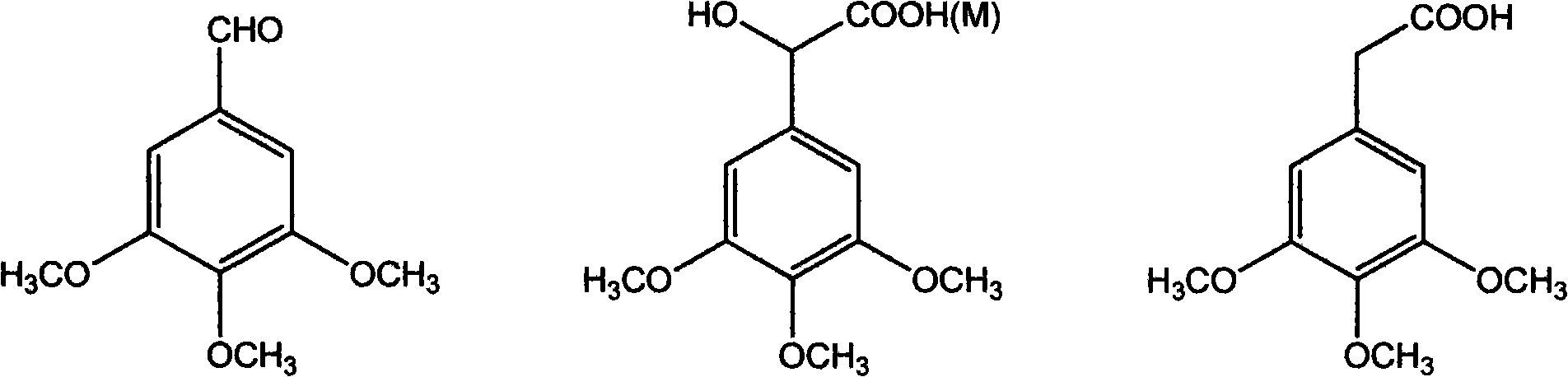
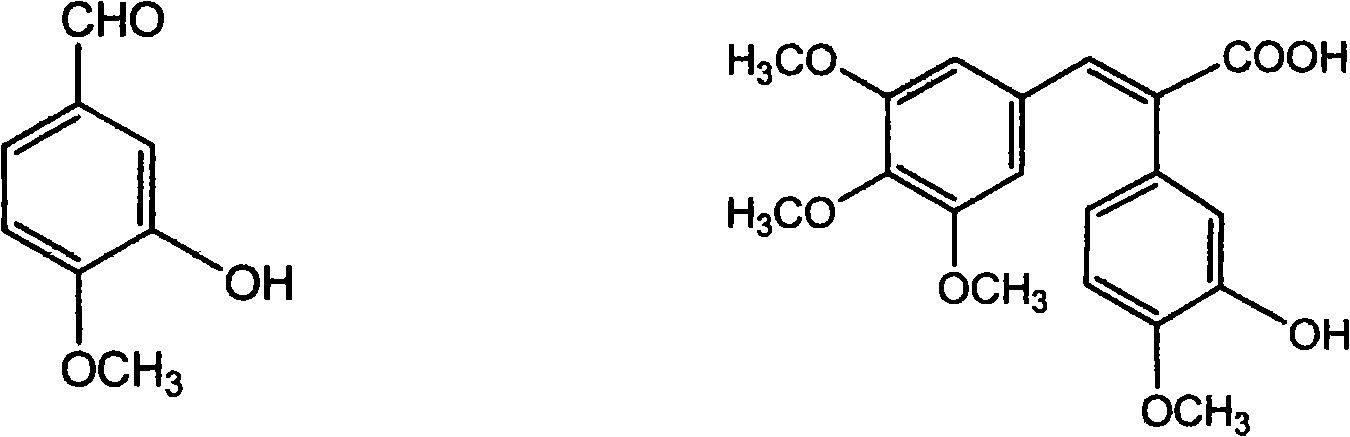
The invention discloses a method for preparing (Z)-3'-hydroxy radical -3,4,4',5-tetra- methoxyl diphenyl ethylene by the material of renewable natural plant resource. Naturally sourced 3,4,5-tri-methoxybenzaldehyde (a derivative extracted from Chinese gall) is taken as the raw material; and 3,4,5-tri-methoyl mandelic acid is obtained through a dichlorocarbene insertion reaction and is reduced to obtain 3,4,5-tri-methoyl phenylacetic acid. The compound can have a Perkin reaction with naturally sourced isovanillin to construct a cis-form diphenyl ethylene backbone, and the (Z)-3'-hydroxy radical -3,4,4',5-tetra- methoxyl diphenyl ethylene is obtained after a decarboxylic reaction. The method adopts the renewable resource-isovanillin and the 3,4,5-tri-methoxybenzaldehyde rich in China to replace increasingly exhausted petrochemical materials, thereby having a good sustainable development capability and remarkable economic, environmental and ecological benefits.
Owner:GUANGZHOU INST OF GEOCHEMISTRY - CHINESE ACAD OF SCI
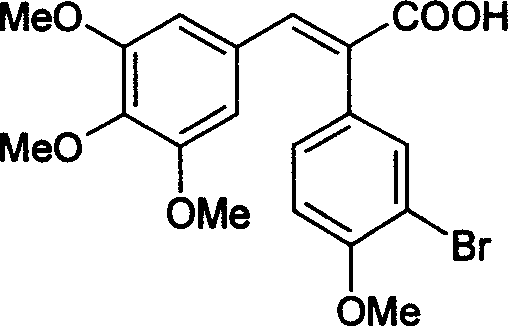
Method for preparing (Z)-3'-hydroxy-3,4,4',5-tetramethoxy diphenyl ethylene from regenerative natural plant resource
InactiveCN101353296AGood sustainable development abilityCost-effectiveOrganic chemistryOrganic compound preparationPetrochemicalMandelic acid
The invention discloses a preparation method for (Z)-3'-hydroxyl-3,4,4',5-tetramethoxyl diphenyl ethylene. A diphenyl ethylene framework structure is built by the Perkin reaction method, and natural aniseed fat-soluble components and propenyl anisole (anethole) are taken as raw materials, and oxidized to obtain anisaldehyde; dichlorocarbene insertion reaction is carried out on the anisaldehyde to obtain p-methoxyl-mandelic acid which is reduced to obtain methoxyl-phenylacetic acid, the methoxyl-phenylacetic acid is brominized to obtain 3-bromo-4-methoxyl-phenylacetic acid. The compound and the natural 3,4,5-trimethoxybenzaldehyde (nutgall extract derivative) carry out Perkin reaction to build a cis-form diphenyl ethylene framework which is further converted and decarboxylated by functional groups to obtain the (Z)-3'-hydroxyl-3,4,4',5-tetramethoxyl diphenyl ethylene. The raw materials of the invention are reproducible natural resources-anethole which are rich in China and 3,4,5-trimethoxybenzaldehyde, and replace non-renewable petrochemical materials which are used by the prior art and become less and less so that the method has the advantages of good sustainable development capability as well as remarkable economic, environmental and ecological benefits.
Owner:GUANGZHOU INST OF GEOCHEMISTRY - CHINESE ACAD OF SCI
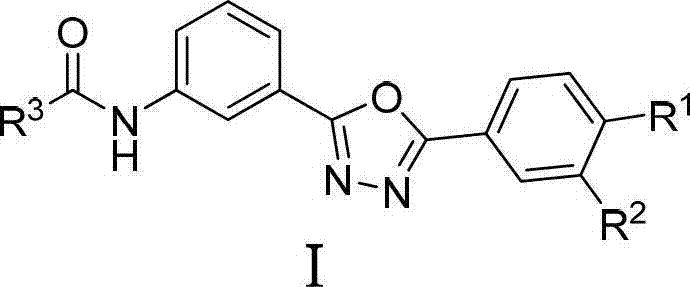
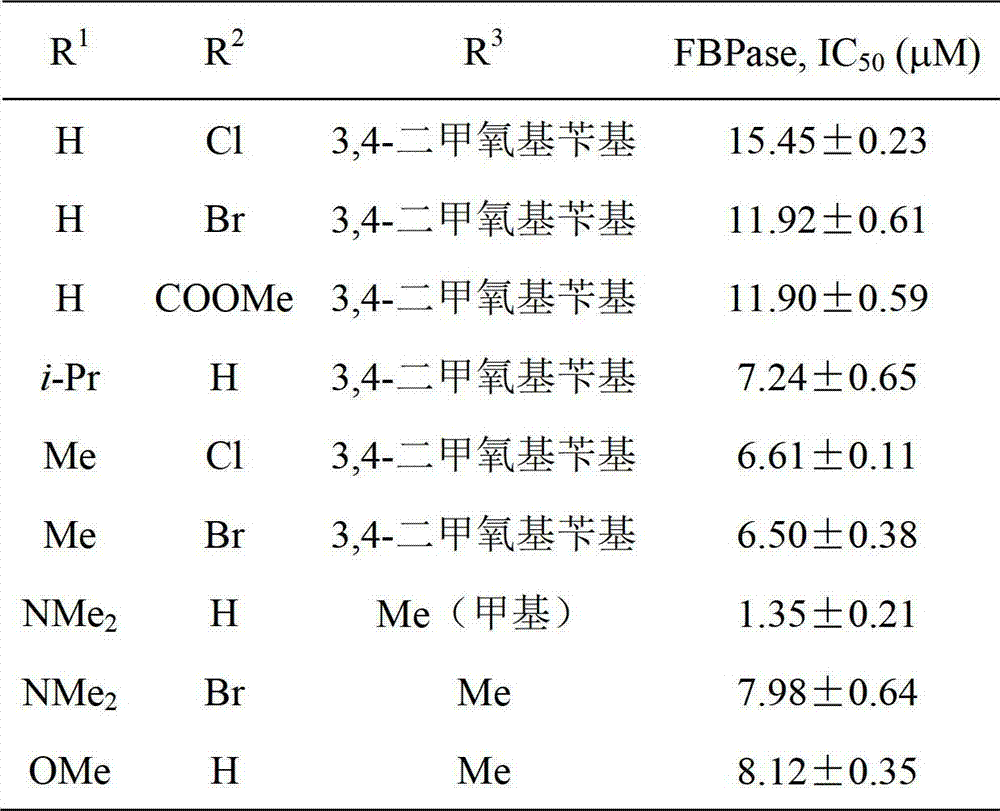
2,5-diaryl-1,3,4-oxadiazole compounds and preparation method and application thereof
InactiveCN102924399AFBPase significantlyReduce the risk of side effectsOrganic active ingredientsOrganic chemistryBenzoic acidAcetic anhydride
The invention relates to 2,5-diaryl-1,3,4-oxadiazole compounds and a preparation method and application thereof. The preparation method comprises the following steps of: condensing benzoic acid of which a 4- site is replaced by R<1> and a 3- site is replaced by R<2> and which is taken as a raw material and m-Nitrobenzoylhydrazine in dichloromethane or tetrahydrofuran to obtain diacylhydrazine i; performing intramolecular dehydration cyclization on the diacylhydrazine i in acetonitrile by taking phosphorus oxychloride as a dehydration reagent to obtain an intermediate ii containing an oxadiazole ring; hydrogenating the intermediate ii under normal pressure under the catalysis of raney nickel to reduce a nitro group to obtain an amino substance iii; and finally, condensing the amino substance iii and acetic anhydride or 3,4-dimethoxyphenylacetic acid under the action of an acid-binding agent to obtain the 2,5-diaryl-1,3,4-oxadiazole compounds I. The invention also discloses application of the 2,5-diaryl-1,3,4-oxadiazole compounds I to the preparation of a medicine for treating type 2 diabetes mellitus.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI +1
Preparation method of COMT inhibitor 5, 6, 7, 3', 4'-pentamethoxyl isoflavone
InactiveCN101643465AEasy to operateShort reaction timeOrganic active ingredientsOrganic chemistrySulfonyl chlorideFormylation reaction
The invention relates to a preparation method for COMT inhibitor 5, 6, 7, 3', 4'-pentamethoxyl isoflavone, comprising the following steps: mixing 3, 4-dimethoxyphenylacetic acid and oxalyl chloride indichloromethane solution to carry out reflux reaction for 2 to 3 h, and cooling to room temperature; adding 3, 4, 5-trimethoxy sodium phenolate to carry out esterification reaction to carry out Friesrearrangement reaction under the catalysis of Lewis acid boron trifluoride ether; and finally carrying out reaction with DMF with the existence of the Lewis acid boron trifluoride ether; and adding methane sulfonyl chloride to promote Vilsmeier-Haack formylation reaction and subsequent cyclization reaction to obtain light yellow crystals 5, 6, 7, 3', 4'-pentamethoxyl isoflavone. The preparation method has simple and easily available raw materials, simple operation, short reaction time, mild reaction condition, environment protection and high yield of 76 %, and is suitable for industrial production.
Owner:DONGHUA UNIV
Preparation method of 2-(4-hydroxyphenyl)-5,7-dimethoxy benzofuran
The invention discloses a synthetic method of 2-(4-hydroxyphenyl)-5,7-dimethoxy benzofuran. The synthetic method comprises the following steps of: firstly, performing bromination reaction and Perkin reaction on 3,5-dimethoxy phenylacetic acid and parahydroxyben-zaldehyde which are used as raw materials to obtain 2-(2-bromo-3,5-dimethoxy phenyl)-3-(4-hydroxyphenyl) acroleic acid; then performing series-wound hydroxylation / intramolecular cyclization / dehydrogenation reaction to obtain 2-(4-hydroxyphenyl)-5,7-dimethoxy benzofuran-3-carboxylic acid; and finally, performing decarboxylic reaction to prepare the 2-(4-hydroxyphenyl)-5,7-dimethoxy benzofuran. According to the method, raw materials are low in price and easy to obtain, the synthetic route is simple, quick and efficient, a noble metal and a ligand are not needed for catalysis, and the method is simple and convenient to operate and good in atom economy.
Owner:GUANGZHOU CHEM CO LTD CHINESE ACADEMY OF SCI
Building paint
InactiveCN105802376AHigh hiding powerImprove adhesionAntifouling/underwater paintsPaints with biocidesPhenyl acetic acidPerillaldehyde
The invention discloses building paint. The building paint is prepared from 3.5-7.5 parts by weight of methyl acetate, 2.3-4.5 parts by weight of hept-2-ene-2-methanol, 6.5-12.7 parts by weight of hydroxymethyl propyl cellulose, 10-15 parts by weight of titanium dioxide, 20-28 parts by weight of white emulsion, 1.2-3.5 parts by weight of perillaldehyde, 2.5-6.4 parts by weight of distyrylphenol polyoxyethylene ether and 3.5-6.8 parts by weight of (R)-(-)-alpha-methoxyphenylacetic acid. Compared with the existing exterior wall paint, the building paint improves an emulsion paint finished product covering capacity through use of a mixture of methyl acetate and white emulsion. Through mixing of hydroxymethyl propyl cellulose and titanium dioxide, latex finished product adhesion and water washing resistance are improved. Through the mixture of perillaldehyde, distyrylphenol polyoxyethylene ether and (R)-(-)-alpha-methoxyphenylacetic acid, the paint film has lasting and beautiful gloss and has lasting pollution resistance.
Owner:QINGDAO JINLIANXIN BUSINESS & TRADE
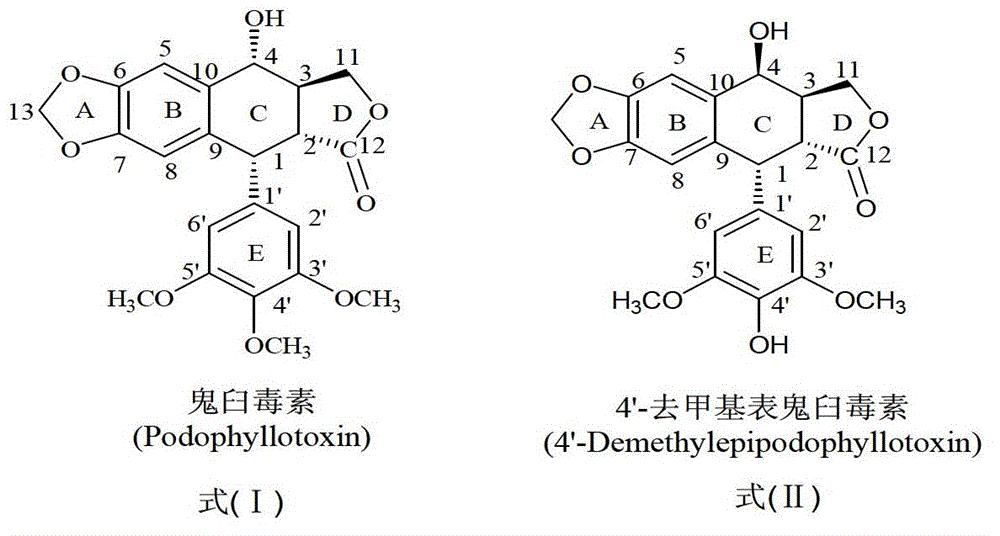
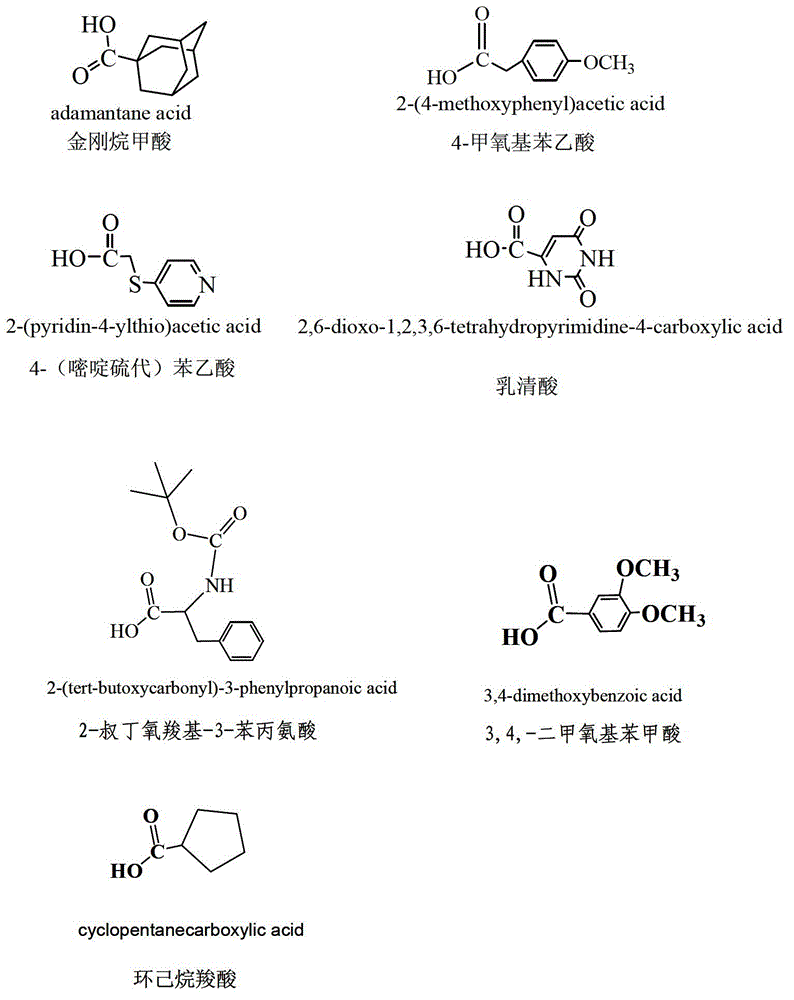
Esterified podophyllum derivative with antitumor activity and preparation method and usage of esterified podophyllum derivative
The invention discloses an esterified podophyllum derivative with antitumor activity and a preparation method and the usage of the esterified podophyllum derivative. The esterified podophyllum derivative with antitumor activity and shown as a formula (V) is obtained by means of esterification reaction of adamantine formic acid, orotic acid, 4-(pyrimidine sulfo) phenylacetic acid, cyclohexane carboxylic acid, 3,4-dimethoxy benzoic acid, 2-butoxycarbonyl-3-phenylalanine or 4-methoxy phenylacetic acid with podophyllotoxin or 4'-demethyl-epipodophyllotoxin. The esterified podophyllum derivative has multi-way multi-target actions on tumor cells to achieve better antitumor curative effects. According to in-vitro cell viability inhibition tests, the esterified podophyllum derivative has excellent antitumor activity and low toxic and side effects and can be prepared into antitumor drugs to be applied to antitumor treatment.
Owner:HUBEI UNIV OF TECH
Preparation method for resveratrol
ActiveCN103664537AShort processEasy to operateOrganic chemistryOrganic compound preparationPhenyl acetic acidAcetic acid
The invention provides a preparation method for resveratrol. The method comprises the following steps: with (3,5-dimethoxyphenyl)acetic acid as a raw material, subjecting (3,5-dimethoxyphenyl)acetic acid and methoxybenzaldehyde dimethyl acetal to a one-pot reaction so as to obtain a crude resveratrol product; and then carrying out neutralizing with alkali lye, decoloring in alcohol and recrystallization so as to prepare a refined resveratrol product. Compared with the prior art, the preparation method provided by the invention has the advantages of short process flow, simple operation and suitability for industrial production, and the prepared refined resveratrol product has yield of more than 93%, a melting point of 261 to 263 DEG C, HPLC of greater than 99%, a whitish color, good quality and low preparation cost.
Owner:HUNAN KEYUAN BIO PRODS
Process for preparing (z)-3'-hydroxy-3, 4, 4', 5-tetranetgixy diphenyl ethylene
InactiveCN1616388AReduce usageRaw materials are cheap and easy to getEther preparationP-methoxyphenylacetic acidPhenol
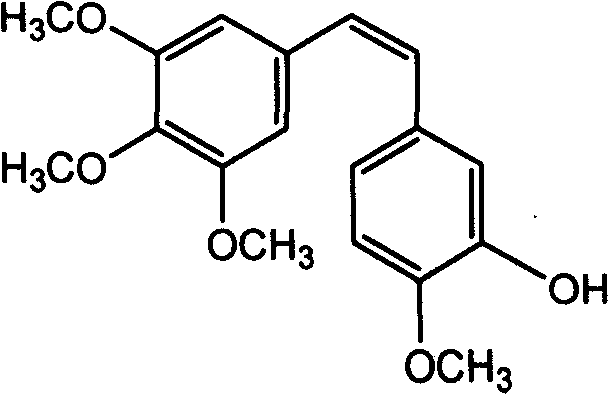
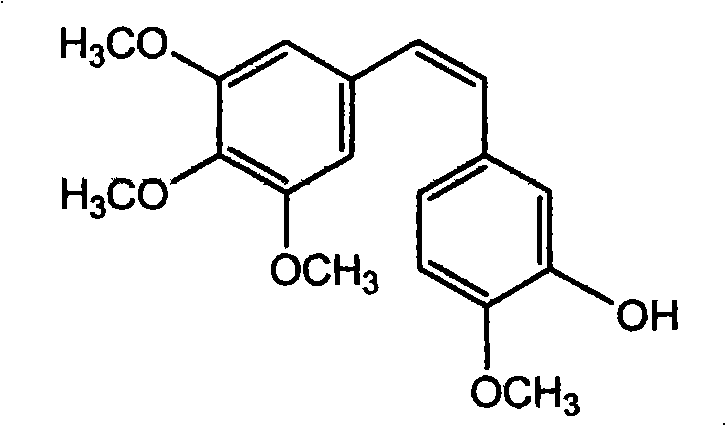
The preparation process of (2)-3'-hydroxy-3, 4, 4', 5-tetramethoxy diphenyl ethylene includes bromizing cheap methoxy phenylacetic acid material to obtain 3-bromo-4-methoxy phenylacetic acid; condensation with 3, 4, 5-trimethoxy phenylaldehyde to obtain (E)-3-(3', 4', 5-trimethoxy phenyl)-2-(3'-bromo-4'-methoxy phenyl)-acrylic acid; phenol hydroxy substitution and debromination to obtain (E)-3-(3', 4', 5-trimethoxy phenyl)-2-(3'-hydroxy-4'-methoxy phenyl)-acrylic acid; and final decarboxyilation to obtain (Z)-3'-hydroxy-3, 4, 4', 5-tetramethoxy diphenyl ethylene. The present invention provides one new design thought of introducing phenol hydroxy radical into diphenyl ethylene compound and one new method of synthesizing (Z)-3'-hydroxy-3, 4, 4', 5-tetramethoxy diphenyl ethylene. The present invention has lowered cost, simplified post-treatment and high total yield.
Owner:GUANGZHOU INST OF GEOCHEMISTRY - CHINESE ACAD OF SCI
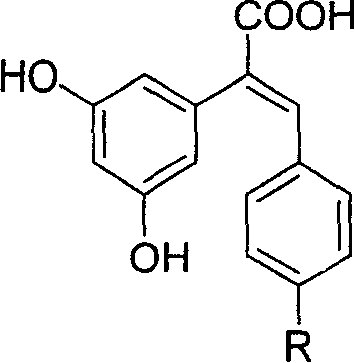
Carboxyl substituted resveratrol analog compound and its preparation method
InactiveCN1807391AEnhance anti-tumorImprove protectionPreparation from nitrilesAntineoplastic agentsHydrogenBenzaldehyde
The resveratrol compound with carboxyl substituent has general formula as following, wherein, R acts for hydrogen, hydroxyl or nitryl. The opposite preparation method comprises: using Perkin reaction to the 3, 5-dimethoxylphenylacetic acid with opposite p-substituting R-benzaldehyde; removing the methoxy protection to obtain the product as the derivative of 1, 2-toluylene. This invention is similar to the resveratrol as 3, 4', 5-trihydroxy -trans-stilbene, and has wide application for antitumor and cardiovascular protection.
Owner:JIANGSU INST OF NUCLEAR MEDICINE
1-carboxy-1-(3,5-dimethoxy phenyl)-2-(4-r group phenyl) ethano and preparation method thereof
InactiveCN1686999AImprove anti-tumor activityImproves antioxidant activityOrganic compound preparationPreparation from nitrilesCarboxyl radicalBenzaldehyde
The present invention relates to a 1-carboxy-1-(3,5-dimethoxyphenyl)-2-(4-R group phenyl) ethylene and its preparation method, belonging to the field of organic chemistry and pharmaceutical chemistry. Said invention provides its general formula. Said preparation method uses 3,5-dimethoxy phenylacetic acid and correspondent para-orientating R group benzaldehyde and makes them produce reaction so as to obtain the invented product which can be used in the medicine field for resisting tumor and resisting oxidation.
Owner:JIANGSU INST OF NUCLEAR MEDICINE
Preparation method of chiral higenamine and derivatives of chiral higenamine
ActiveCN103626703AHigh selectivityHigh purityOrganic chemistryBulk chemical productionCombinatorial chemistryPerylene derivatives
The invention discloses a preparation method of chiral higenamine. The chiral higenamine is prepared from 4-methoxyphenylacetic acid by the following steps: chlorination, acylation and asymmetric reduction, cyclization, asymmetric reduction, removal of protecting groups and the like. The preparation method has the advantages of high selectivity, simplicity, efficiency and the like. The ee. value of the product is over 95%, the purity of the product is high, and the total yield can reach 30%. Production and preparation in a large scale can be realized.
Owner:珠海润都制药股份有限公司
Full-synthetic method of racemic tetrandrine
ActiveCN109942593ALow costHigh synthesis efficiencyOrganic chemistryBulk chemical productionDrugs synthesisSynthesis methods
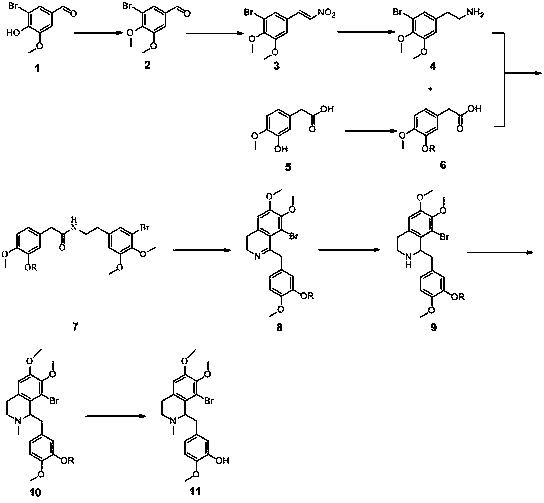
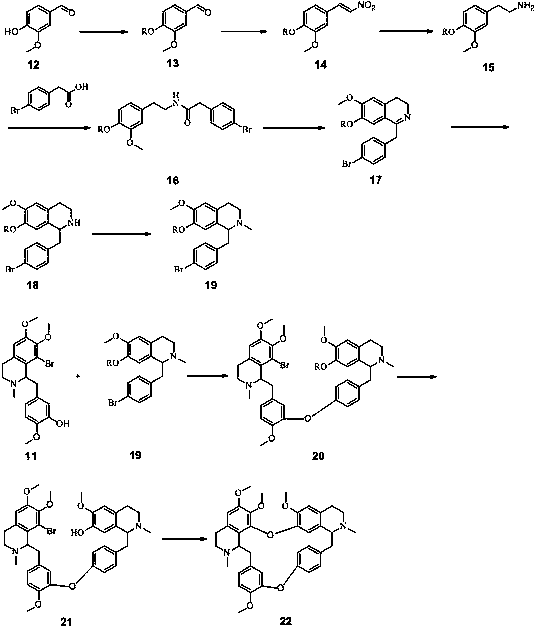
The invention discloses a full-synthetic method of racemic tetrandrine, and belongs to the technical field of drug synthesis. The full-synthetic method of the racemic tetrandrine comprises the steps that a synthetic route adopts a convergence synthesis method, a 5-bromovanillin, namely a compound 1 and 3-hydroxy-4-methoxyphenylacetic acid, namely a compound 5 are taken as starting materials to obtain a compound 4 and a compound 6 respectively, then the compound 4 and the compound 6 are taken as raw materials to synthesize a compound 11, a compound 19 is synthesized from the compound 11, and finally, the compound 11 reacts with the compound 19. The full-synthetic method of the racemic tetrandrine has the advantages that the synthetic efficiency is higher, the yield is higher, and the cost is lower; the reaction conditions are milder, the operation is simple and convenient, the industrial value is higher, and a reference is provided for the full-synthetic method of optical voidness tetrandrine.
Owner:ZHEJIANG JINHUA CONBA BIO PHARM CO LTD +1
Preparation method for ivabradine impurities
InactiveCN108424389AImprove medication safetyEasy to prepareOrganic chemistryBulk chemical productionIvabradineImpurity
The invention discloses a preparation method for ivabradine impurities. According to the preparation method, 3-hydroxy-4-methoxyphenylacetic acid (as shown in a formula 1a) and 4-hydroxy-3-methoxyphenylacetic acid (as shown in a formula 1b) are respectively used as a starting raw material and subjected to multiple steps of reactions to prepare two impurities of ivabradine. The preparation method of the invention is simple and realizes high-purity preparation; and the prepared impurities can be used for qualitative and quantitative analysis so as to improve the medication safety of ivabradine.
Owner:ZHEJIANG JINGXIN PHARMA
Synthesis method of Formononetin
InactiveCN102070593ASolve insufficient resourcesRaw materials are easy to getOrganic chemistrySodium bicarbonateSynthesis methods
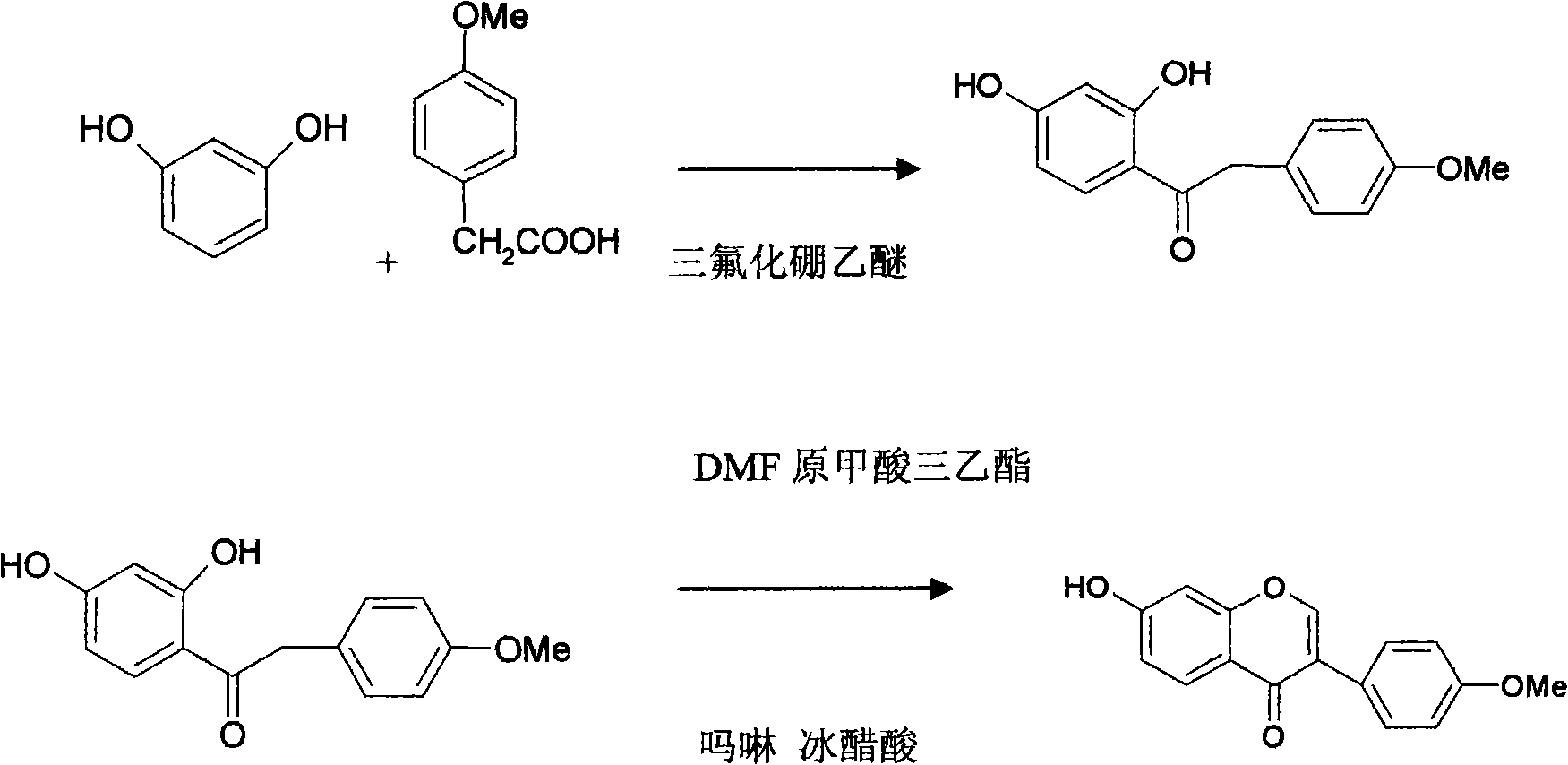
The invention relates to a synthesis method of formononetin, and the method comprises the following steps: condensation: in a dry reactor, adding resorcin, p-metoxybenzene acetate, boron trifluoride and ethyl ether, heating for reaction, adding water after the reaction finishes, heating under reflux, cooling to precipitate crystals, filtering, washing with water until the solution is neutral, and drying to obtain an intermediate; and cyclization: in a dry reaction tank, adding the intermediate, triethyl orthoformate, morpholine, DMF (dimethyl formamide) and glacial acetic acid, separating out the reaction byproduct ethanol, heating under reflux, recycling the DMF reagent under reduced pressure after the reaction finishes, cooling, adding saturated sodium bicarbonate solution into the remainder, refluxing, cooling to precipitate crystals, carrying out conventional treatment to obtain crude formononetin, and refining the crude formononetin by using methanol to obtain the fine formononetin. The method can be used for solving the problem of formononetin resource shortage at present, has the advantages of accessible raw materials, efficient reaction, convenience and high application value.
Owner:SHAANXI JIAHE PHYTOCHEM
Preparation method of 7,8-dimethoxy-1,3-dihydro-2h-3-benzazepin-2-one
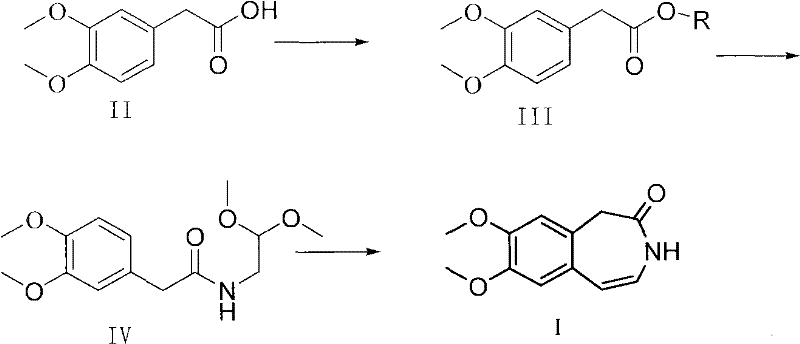
The invention belongs to the field of medicinal preparations and provides a method for preparing a key intermediate of ivabradine, namely 7,8-dimethoxy-1,3-dihydro-2H-3-benzazepin-2-one. The method is characterized in that: a form of active ester is obtained by activating a carboxyl group of 3,4-dimethoxyphenylacetic acid under the catalysis of alkali, and then the active ester and 2,2-dimethoxyethylamine are ammoniated and cyclized to form a target product, so that the conditions such as adoption of thionyl chloride, high-temperature distillation and the like which are not suitable for industrialized production are avoided, the yield of the reaction is remarkably improved and reaches 75 to 80 percent, and the method is more suitable for large-scale industrialized production.
Owner:QILU PHARMA CO LTD
Preparation method for lamellar inorganic-organic composite energy storage and conversion material
The invention belongs to the field of preparation of energy storage and conversion materials and particularly relates to a preparation method for a lamellar inorganic-organic composite energy storage and conversion material, which uses lamellar hydrotalcite-like compounds as a main body and uses a fluorescent chromophore with donor-receptor properties as an object. The preparation method comprises the steps that the lamellar inorganic-organic composite energy storage and conversion material is prepared by the hydrotalcite-like compounds and the fluorescent chromophore through the ion exchange reaction in ethylene glycol dispersion media. The hydrotalcite-like compounds are magnalium hydrotalcite, zinc-aluminum hydrotalcite or cobalt-aluminum hydrotalcite, the organic fluorescent chromophore comprises ethyl orange, 4-amino azo phenyl-4 sulfonate, 4-methoxyphenylacetic acid, 2-naphthalene sulfonic acid and coumarin-3-formic acid or 9-anthracenecarboxylic acid. A prepared product has a lamellar structure and specific fluorescence storage and conversion performance. The invention aims at developing the preparation method for the lamellar inorganic-organic composite energy storage and conversion material and adopts the ion exchange reaction so that three wastes including waste gas, waste water and waste residues are not produced in the preparation process.
Owner:BEIJING UNIV OF CHEM TECH
Plasma metabolization micromolecule marker related to human non-small-cell lung cancer and application of plasma metabolization micromolecule marker
InactiveCN105203683ARepair inhibitionIncrease contractilityComponent separationMetaboliteConfidence interval
The invention belongs to the field of analytical chemistry and clinical medicine and relates to a plasma metabolization micromolecule marker related to the human non-small-cell lung cancer and an application of the plasma metabolization micromolecule marker. The plasma metabolization micromolecule marker related to the human non-small-cell lung cancer is one or more of cortisol, corticosterone and 4-methoxyphenylacetic acid. The plasma metabolization micromolecule marker is prepared from cortisol, corticosterone and 4-methoxyphenylacetic acid. The content range (95% confidence interval) of cortisol is 0.00018-0.00067, the content range (95% confidence interval) of corticosterone is 0.000029-0.00010, the content range (95% confidence interval) of 4-methoxyphenylacetic acid is 0.000015-0.000022, and metabolite can prompt occurrence of tumors within the ranges. The horizontal range, corresponding to a normal group, of cortisol is 0.0030-0.0037, the horizontal range, corresponding to the normal group, of corticosterone is 0.00044-0.00056, and the horizontal range, corresponding to the normal group, of 4-methoxyphenylacetic acid is 7.39 E-07-2.09 E-06. The plasma metabolization micromolecule marker is a novel biomarker, compared with a traditional protein biomarker, the relevance between the marker and the disease outcome is higher, and the plasma metabolization micromolecule marker is stable, minimally invasive, easy to detect and accurate in quantitation.
Owner:JIANGSU PROVINCE HOSPITAL
A kind of synthetic method of methoxyphenamine hydrochloride
InactiveCN102267917AGreenEasy to industrializeOrganic compound preparationChemical recyclingAcetic anhydrideHigh pressure
The invention provides a method for synthesizing methoxyphenamine hydrochloride. The method comprises the following steps of: (1) mixing o-methoxyphenylacetic acid with organic alkali, dissolving in acetic anhydride for undergoing a heating reaction, and performing post-treatment to obtain o-methoxybenzyl methyl ketone; (2) putting the o-methoxybenzyl methyl ketone and a methylamine alcohol solution into a high-pressure kettle for performing catalytic hydrogenation to obtain o-methoxy phenpromethamine; and (3) introducing dried HCl gas into a solution containing o-methoxy phenpromethamine forsalifying, and recrystallizing the obtained crude product to obtain methoxyphenamine hydrochloride. The method has the advantages of easiness and convenience for operation, mild reaction condition, high reaction yield, small quantity of three wastes, lower raw material cost, easiness for industrialization, and the like.
Owner:ZHEJIANG UNIV OF TECH +1
Preparation method of (R,S)-2-[[5-(9-fluorenemethoxycarbonylamino)dibenzo[A,D]cycloheptane-2-yl]oxyl]acetic acid
ActiveCN104761470AEasy post-processingReduce manufacturing costCarbamic acid derivatives preparationOrganic compound preparationBenzoic acidEvaporation
The invention relates to a preparation method of Ramage linker and mainly solves problems of long processes, complex post-treatment, much waste water, waste gas and solid waste, and high cost in a conventional synthetic method. The preparation method includes following steps: (A) carrying out a reaction to 2-carboxybenzaldehyde and m-methoxyphenylacetic acid to obtain an intermediate 2-(3-methoxylstyryl)benzoic acid, dissolving the intermediate with a solvent, performing hydrogenation reduction, and performing post-treatment crystallization to obtain a compound R-1; (B) carrying out a reaction to the R-1 with SOCl2 or POCl3 to obtain 2-methoxyl-10,11-dihydro-5H-dibenzo[a,d]cycloheptene-5-one, performing negative-pressure evaporation to remove the SOCl2 or the POCl3, dissolving the 2-methoxyl-10,11-dihydro-5H-dibenzo[a,d]cycloheptene-5-one in benzene, methylbenzene or 1,2-dichloroethane, performing a catalytic reaction with anhydrous AlCl3 and performing post-treatment crystallization to obtain a compound R-2; (C) carrying out a reaction to the R-2 with benzyl bromoacetate in DMF or an acetone / K2CO3 solution to obtain a compound R-3; (D) performing hydrogenation reduction to the R-3 to obtain a compound R-4; and (E) adding a catalyic amount of PTS to the R-4 in DMF and carrying out a reaction to the R-4 with Fmoc-NH2 to obtain the Ramage linker, which is an effective C-terminal linker in solid-phase synthesis.
Owner:江苏吉泰肽业科技有限公司
Synthesis method of elagolix intermediate
ActiveCN110759870AReduce usageRaw materials are cheap and easy to getOrganic chemistryBiochemical engineeringCombinatorial chemistry
The invention relates to a synthesis method of an elagolix intermediate. Specifically, the invention provides a synthesis method of an elagolix intermediate compound X and an elagolix intermediate compound I. According to the method, 2-fluoro-3-methoxy-phenylacetic acid is used as a raw material, and the steps of cyclization, hydrolysis, amino protection, condensation, Mitsunobu reaction and the like are carried out in sequence, so that the elagolix intermediate compound X and the elagolix intermediate compound I are obtained. The method has the advantages of cheap and easily available reagents, high conversion rate, simple operation and low process cost, and is suitable for industrialization.
Owner:SHANGHAI VASTPRO TECH DEV CO LTD
Preparation method of 3, 4-dimethoxybenzonitrile
ActiveCN110668972AImprove securitySimple stepsPreparation by nitrogen oxide-organic compound reactionNitric oxidePtru catalystNitrogen source
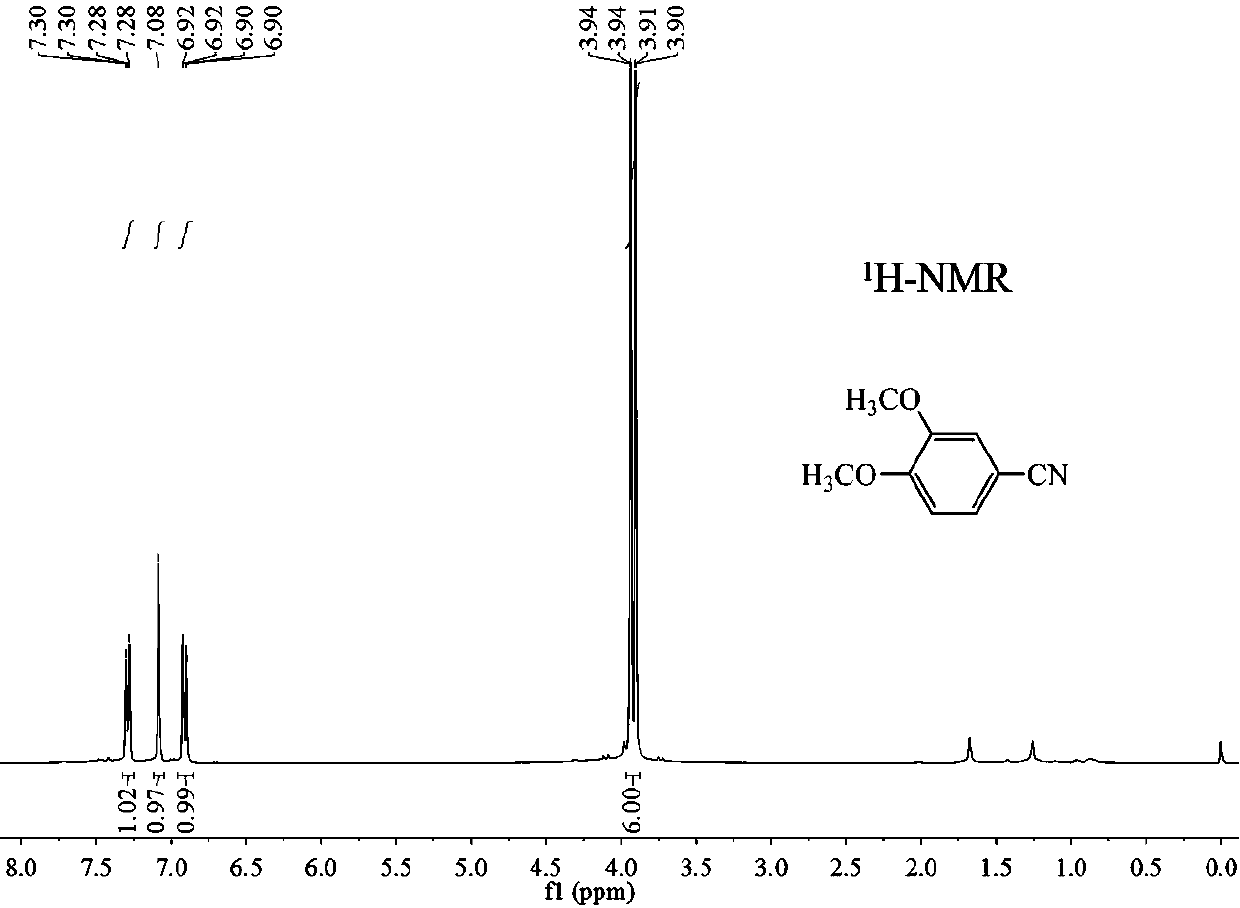
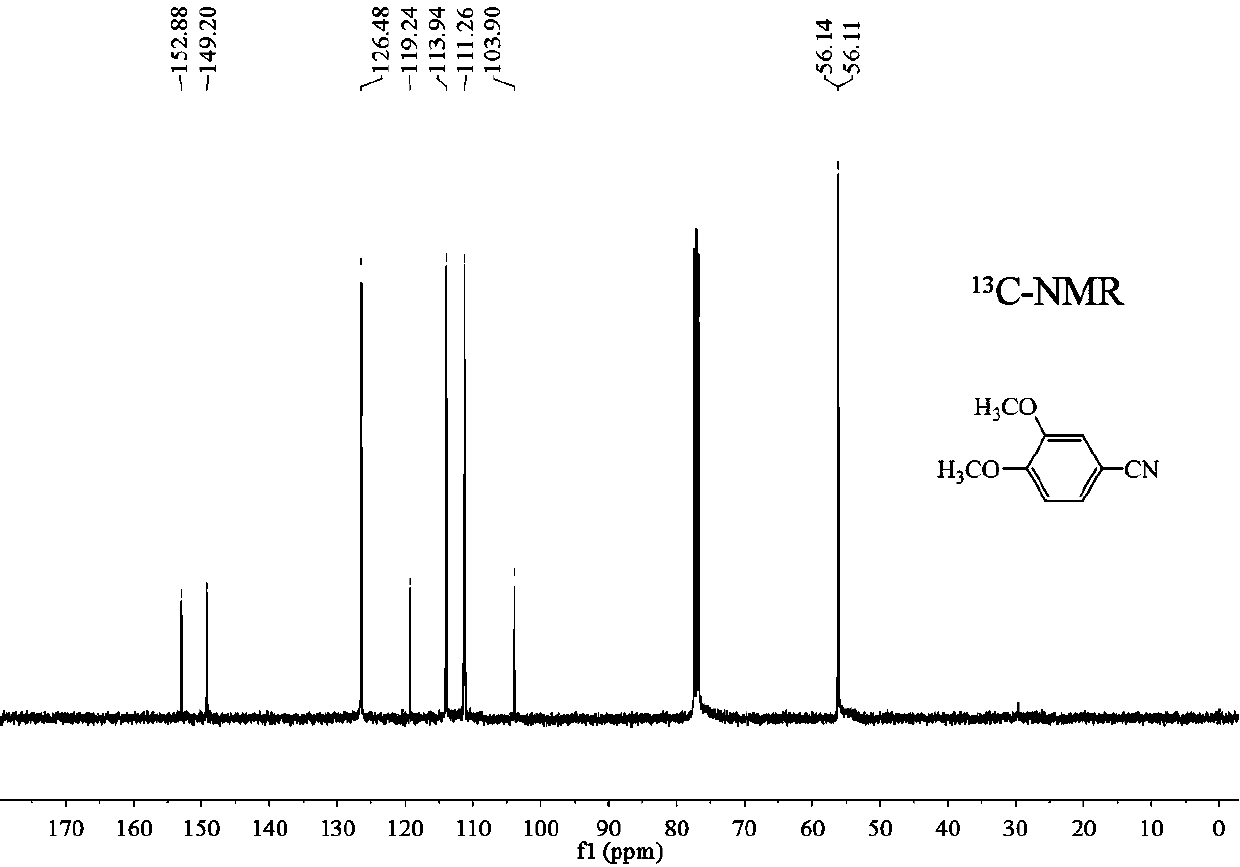
A preparation method of 3,4-dimethoxybenzonitrile is characterized in that 3,4-dimethoxyphenylacetic acid and sodium nitrite are used as raw materials, and ferric trichloride is used as a catalyst. The preparation method which is different from previous synthesis methods has the following characteristics: the used raw materials are cheap and easily available phenylacetic acid compounds, and the sodium nitrite is used as a nitrogen source, so the toxicity of the raw materials is low, and the price is low. The problem of high comprehensive cost of materials such as reaction reagents used in theexisting method is solved. The preparation method of the 3,4-dimethoxybenzonitrile has the advantages of low toxicity, simple process, mild conditions and high finished product yield.
Owner:河南睿嵩检测技术有限公司
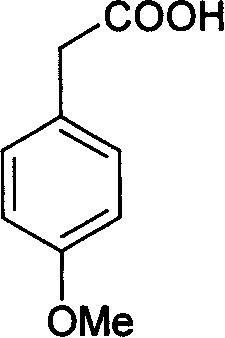
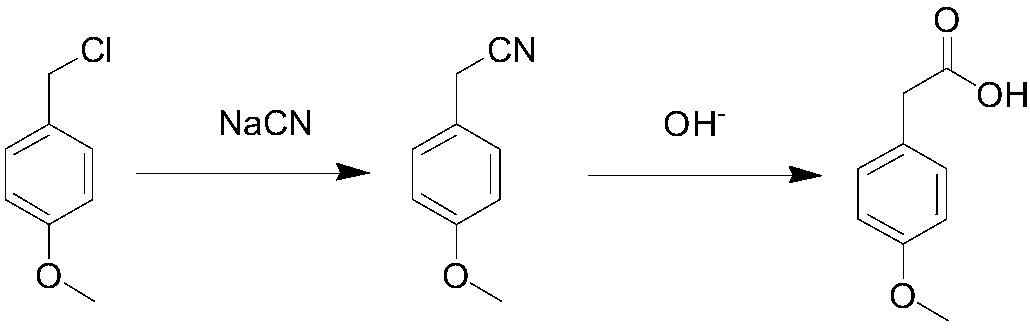
Method for synthesizing p-methoxyphenylacetic acid
InactiveCN108191633AEasy to storeLow raw material costOrganic compound preparationCarboxylic acid esters preparationEcological environmentMalonate
The invention discloses a method for synthesizing p-methoxyphenylacetic acid. The method comprises the following steps: adding malonate, alkali and p-methoxy halobenzene in a solvent, enabling the p-methoxy halobenzene and the malonate to perform alkylation reaction under the alkali condition, adding water to perform quenching reaction after the reaction is finished, directly acidizing and heatingto perform the decarboxylation hydrolysis reaction after concentrating out most solvent, and then cooling to crystallize, filter and dry, namely obtaining the p-methoxyphenylacetic acid. The method disclosed by the invention has the following advantages: 1, the raw materials used by the method of the invention are easier to obtain and convenient for storing, the raw material and operation cost are greatly reduced; 2, the method disclosed by the invention is less in reaction step, the intermediate reaction process is easy to control and easy for scale production; and 3, the three waste (wastewater, waste solid and waste gas) yield is less, the environment pollution is reduced, and the ecological environment is protected. And meanwhile, the prepared product is high in purity, and the purity can achieve 99% or more; the yield is high and can achieve 95% or more.
Owner:抚顺东科新能源科技有限公司
Method for preparing (Z)-3'-hydroxy-3,4',5-trimethoxy diphenylethene
InactiveCN101665419ALow priceLow costOrganic chemistryOrganic compound preparationHydrolysisMedicinal chemistry
The invention discloses a method for preparing (Z)-3'-hydroxy-3,4',5-trimethoxy diphenylethene, comprising the following steps: taking 3,5-dihydroxyacetophenone as raw material, and then obtaining 3,5-dimethoxy hypnone by methylation reaction under the alkaline condition; then obtaining 3,5-dimethoxyphenylacetic acid by Willgerodt-Kindler rearrangement and hydrolysis reaction; enabling the 3,5-dimethoxyphenylacetic acid and isovanillin to carry out Perkin reaction to obtain (E)-2-(3',5'-dimethoxyphenyl)-3-(3'-hydroxy-4'-methoxyphenyl)crylic acid, and then obtaining the target compound, i.e. the (Z)-3'-oxhydryl-3,4',5-trimethoxy diphenylethene, by decarboxylic reaction. The raw materials of 3,5-dihydroxyacetophenone and isovanillin adopted by the invention have low price and are easy to obtain. The technical process has the advantages of simple operation, good cis-selectivity and high yield.
Owner:中科检测技术服务(广州)股份有限公司
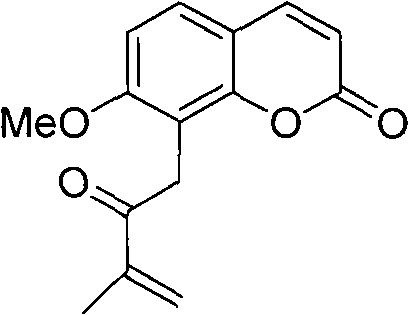
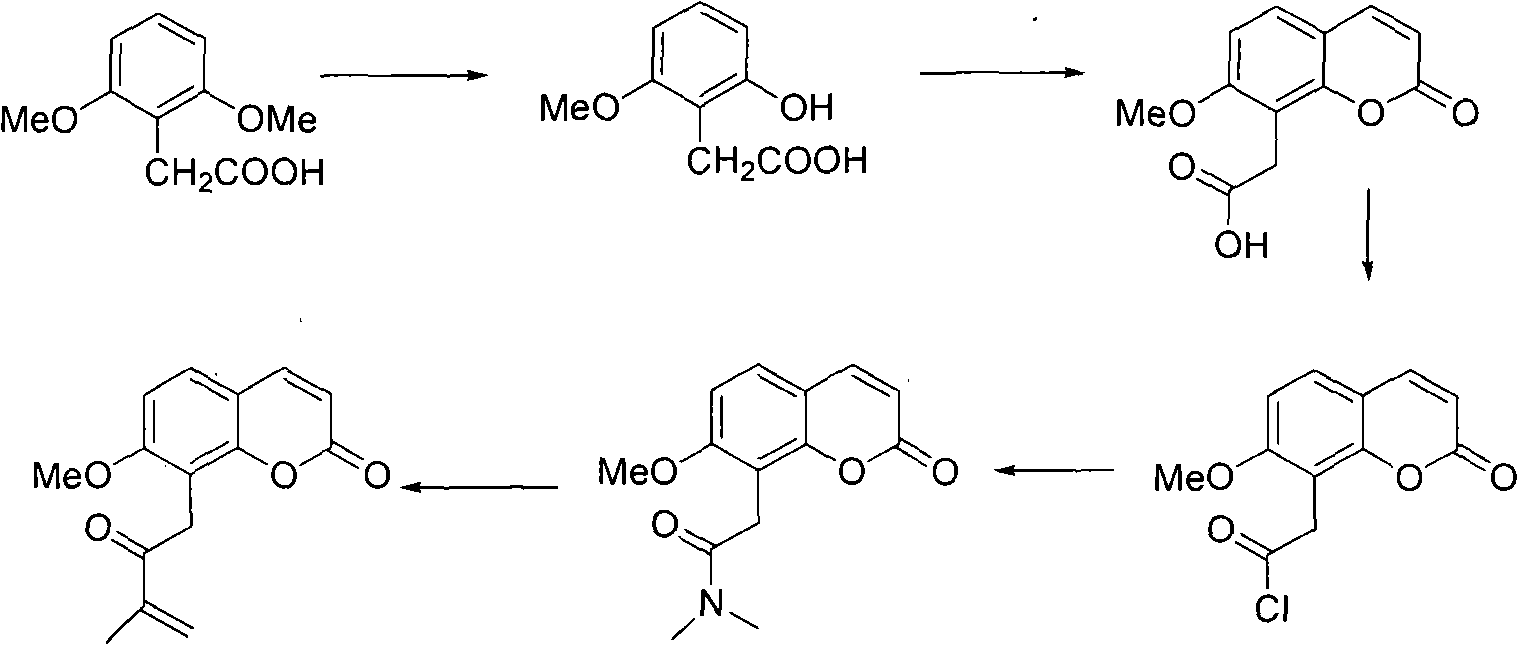
Novel synthesis method of murrayone and novel application of murrayone
InactiveCN102603694ARaw materials are easy to getMild reaction conditionsOrganic chemistrySkeletal disorderSynthesis methodsGrignard reagent
The invention relates to the technical field of pharmaceuticals, in particular to a novel synthesis method of murrayone and novel application of murrayone in the preparation of drugs for preventing and treating osteoarthritis. The novel synthesis method of murrayone comprises the following steps: reacting 2,6-dimethoxyphenylacetic acid with boron tribromide to prepare 2-hydroxy-6-methoxyphenylacetic acid; reacting the 2-hydroxy-6-methoxyphenylacetic acid with ethyl propiolate to prepare 7-methoxy-8-acetoxy coumarin; reacting the prepared 7-methoxy-8-acetoxy coumarin with solid phosgene to prepare an acyl chloride compound at first, then adding dimethylamine to prepare 7-methoxy-8-(N,N-dimethyl acetamido) coumarin; and reacting the prepared 7-methoxy-8-(N,N-dimethyl acetamido) coumarin with a Grignard reagent such as isopropenyl magnesium bromide to prepare murrayone. Compared with the prior art, the required raw materials are easily accessible, the reaction conditions are mild, and the operation is simple.
Owner:GANNAN MEDICAL UNIV
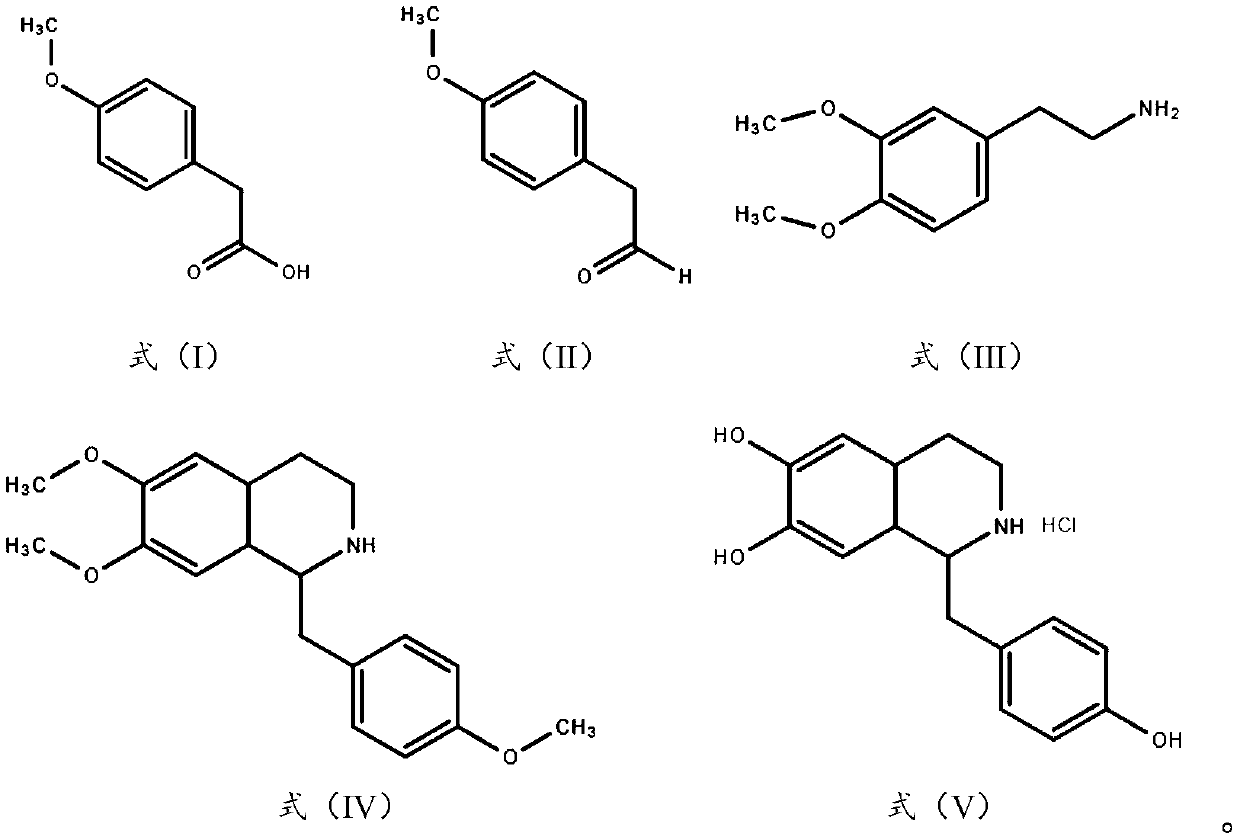
Synthesis technology of higenamine and pharmaceutical salt of higenamine
The invention discloses a synthesis technology of higenamine and pharmaceutical salt of the higenamine, and belongs to the technical field of medicine synthesis. According to the synthesis technology,4-methoxyphenylacetic acid is used as a raw material, and through steps of reduction, oxidation, cyclization, deprotection and the like, the higenamine and the hydrochloride of the higenamine are obtained. Compared with existing technical routes, the synthesis technology has the few steps, and is simple to operate, the raw material is cheap, reaction conditions are mild, a reaction process is easy to control, the total yield and the purity are high, and the synthesis technology is environmentally friendly, safe and suitable for industrial production, and has large industrial prospects.
Owner:珠海市柏瑞医药科技有限公司
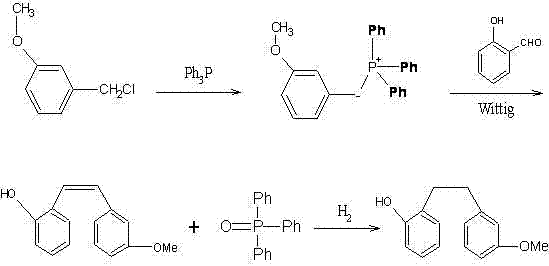
Preparation method of Sarpogrelate intermediate 2-((3-methoxy) phenethyl) phenol
InactiveCN102516043ASolve pollutionSimple processOrganic chemistryOrganic compound preparationAcetic anhydrideSalicylaldehyde
The invention aims to provide a preparation method of Sarpogrelate intermediate 2-((3-methoxy) phenethyl) phenol (I), which comprises the following steps of: 1) carrying out perkin reaction on 3-methoxyphenylacetic acid (II), salicylic aldehyde (III), organic base and acetic anhydride; 2) further obtaining 2-(3-methoxyphenyl)-3-(2-acetoxy-phenyl) acrylic acid (IV); 3) decarboxylating a compound IV under the existence of quinoline and metal copper to obtain 3-methoxy-2'-acetoxy stilbene (V); 4) hydrolyzing the compound IV to obtain 2-((3-methoxy) styryl) phenol (VI); and 5) carrying out catalytic hydrogenation on the compound IV to obtain a compound I. The technical scheme disclosed by the invention has the characteristics of simplicity in operation, mild reaction condition, no hydroxyl protection or deprotection required, easiness for product purification, short synthetic route, easiness for material obtaining, high yield, low cost and the like and has a wide industrial application prospect.
Owner:PKU HEALTHCARE CORP LTD
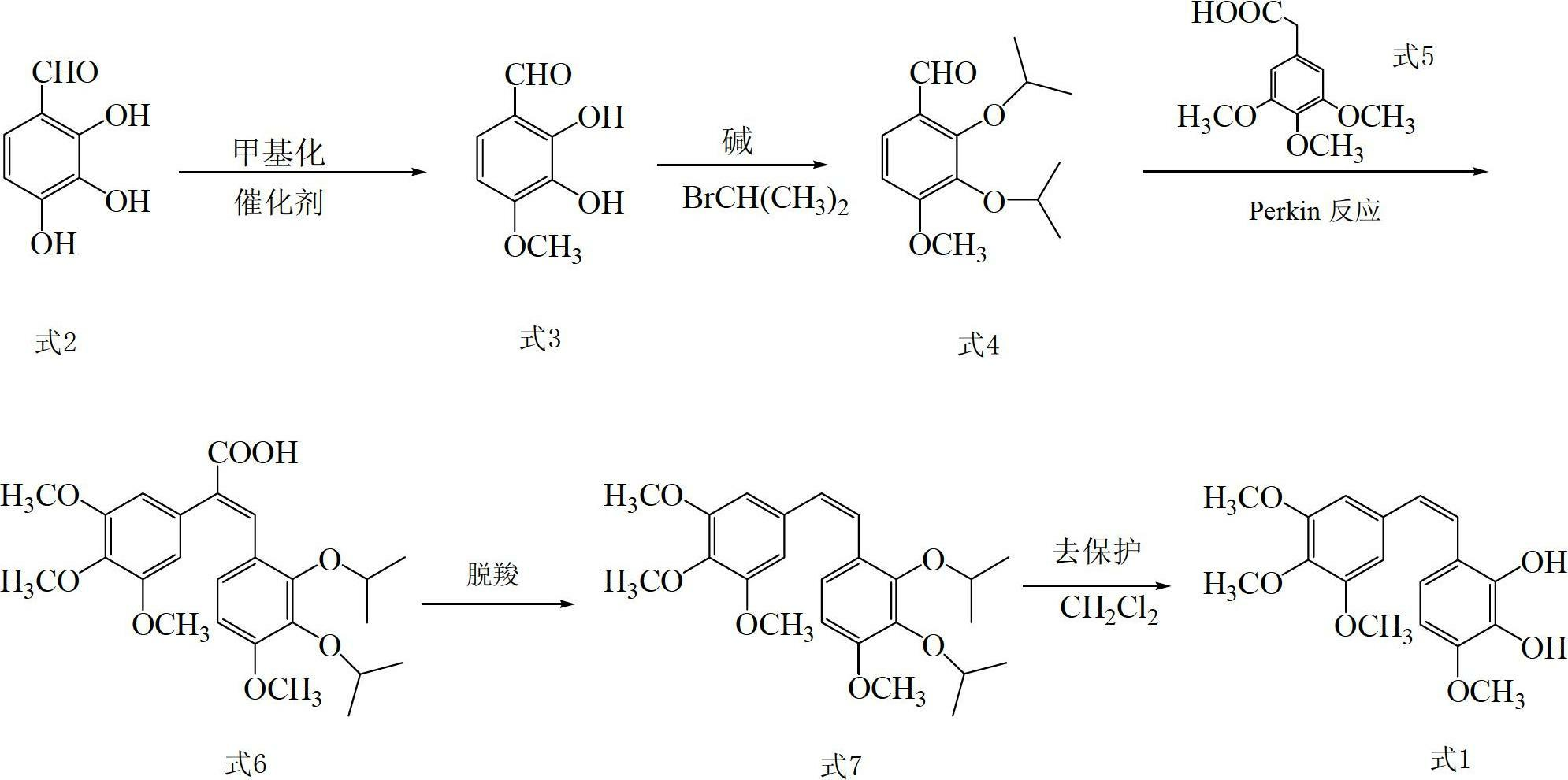
Preparation method and application of Z-3,4,4',5-tetramethoxy-2',3'-dihydroxy diphenylethylene
The invention discloses a preparation method and application of Z-3,4,4',5-tetramethoxy-2',3'-dihydroxy diphenylethylene. The preparation method comprises the following steps of: taking 2,3,4-trihydroxy phenylfluorone as a raw material; carrying out single methylation on the 2,3,4-trihydroxy phenylfluorone; carrying out substitution reaction on the methylated 2,3,4-trihydroxy phenylfluorone with bromopropane so as to obtain 2,3-diisopropoxy-4-methoxybenzaldehyde; carrying out Perkin reaction on 2,3-diisopropoxy-4-methoxybenzaldehyde with 3,4,5-trimethoxyphenylacetic acid so as to obtain E-2-(3,4,5-trimethoxyphenyl)-3-(2',3'-diisopropoxy-4'-methoxyphenyl)acrylic acid; carrying out decarboxylation to obtain Z-3,4,4',5-tetramethoxy-2',3'-diisopropoxy diphenylethylene; and finally obtaining Z-3,4,4',5-tetramethoxy-2',3'-dihydroxy diphenylethylene after carrying out deprotection. The synthetic route provided by the invention is simple and direct, cis-selectivity is higher, and the yield of the Perkin reaction as well as the total yield of Z-3,4,4',5-tetramethoxy-2',3'-dihydroxy diphenylethylene are both higher. The preparation method disclosed by the invention has the characteristics of simpleness and feasibility for operation, low price and easy obtainment of used raw materials and reagents, small pollution on environment, good atom economy, low cost and capability of being applied to industrialized production.
Owner:GUANGZHOU CHEM CO LTD CHINESE ACADEMY OF SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com