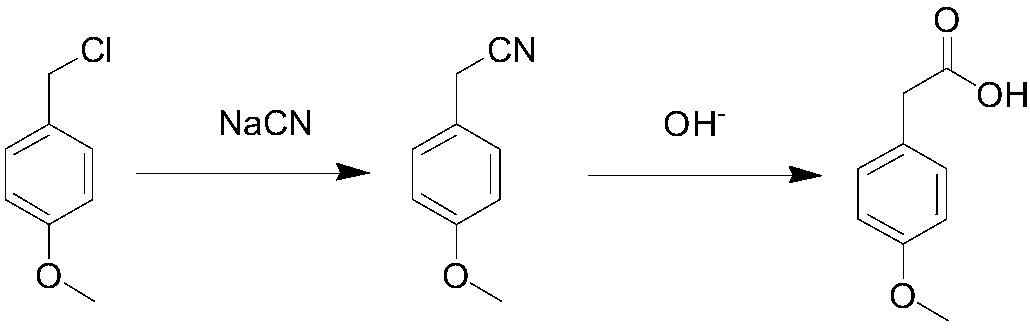
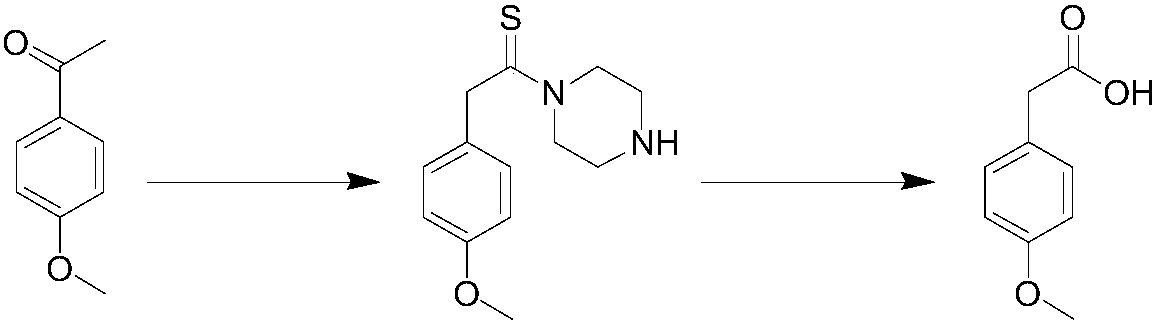
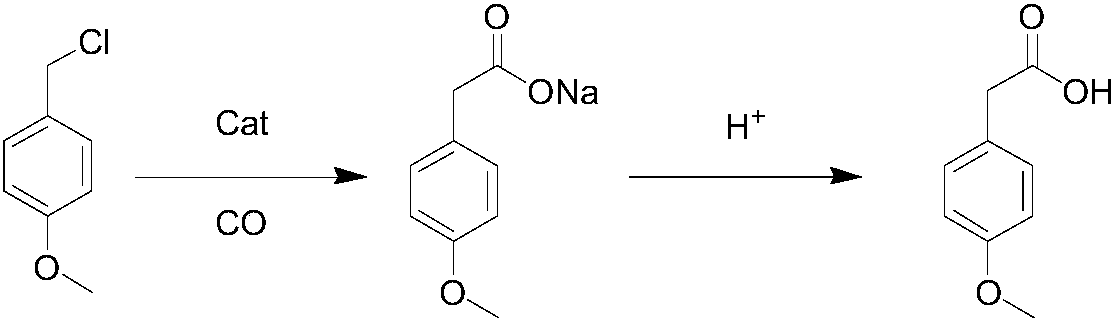
Method for synthesizing p-methoxyphenylacetic acid
A technology of p-methoxybenzene and methoxyhalobenzene, which is applied in the field of synthesizing p-methoxyphenylacetic acid, can solve the problems of high requirements for reaction equipment and expensive catalysts, achieve low output of three wastes, reduce raw materials and operating costs , the effect of protecting the ecological environment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Example 1: Add 61 g (1.0 eq) of tert-butyl methyl malonate and 300 milliliters of tetrahydrofuran to the reaction flask under the protection of argon, and add 8.4 g (1.0 eq) of sodium hydride in batches under an ice bath. After the addition is completed, rise to React at room temperature for 1 hour, then slowly add a tetrahydrofuran solution of p-methoxychlorobenzene (50g, 1.0eq) dropwise below 20°C, and the dropwise addition is completed in about 50 minutes. , add 30 ml of water to quench the reaction, concentrate to remove most of the solvent, add 200 ml of concentrated hydrochloric acid, heat up to 100 degrees again and stir the reaction for 16 hours, concentrate to remove most of the water, slowly cool down to crystallize, filter to obtain p-methoxybenzene Acetic acid 49.5g, yield 85%, HPLC: 99.3%.
Embodiment 2
[0036] Example 2: Add 46.6 g (1.0 eq) of tert-butyl methyl malonate and 300 milliliters of tetrahydrofuran to the reaction flask under the protection of argon, and add 6.4 g (1.0 eq) of sodium hydride in batches under an ice bath. React at room temperature for 1 hour, slowly add a tetrahydrofuran solution of p-methoxybromobenzene (50g, 1.0eq) dropwise below 20°C, and complete the dropwise addition in about 50 minutes. After the drop, raise the temperature to 65°C for 10 hours, then cool , add 30 ml of water to quench the reaction, concentrate to remove most of the solvent, add 200 ml of concentrated hydrochloric acid, heat up to 100 degrees again and stir the reaction for 16 hours, concentrate to remove most of the water, slowly cool down to crystallize, filter to obtain p-methoxybenzene Acetic acid 42.6g, yield 96%, HPLC: 99.6%.
Embodiment 3
[0037] Example 3: Add 50.3 g (1.0 eq) of tert-butyl ethyl malonate and 300 milliliters of tetrahydrofuran to the reaction flask under the protection of argon, and add 6.4 g (1.0 eq) of sodium hydride in batches under an ice bath. , raised to room temperature and reacted for 1 hour, slowly added dropwise a tetrahydrofuran solution of p-methoxybromobenzene (50g, 1.0eq) below 20 degrees, and the dropwise addition was completed in about 50 minutes. After the drop, the temperature was raised to 65 degrees for 10 hours , cool down, add 30 ml of water to quench the reaction, concentrate to remove most of the solvent, add 200 ml of concentrated hydrochloric acid, heat up to 100 degrees again and stir the reaction for 16 hours, concentrate to remove most of the water, slowly cool down to crystallize, filter to obtain p-methoxy Phenylacetic acid 42.0g, yield 95%, HPLC: 99.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com