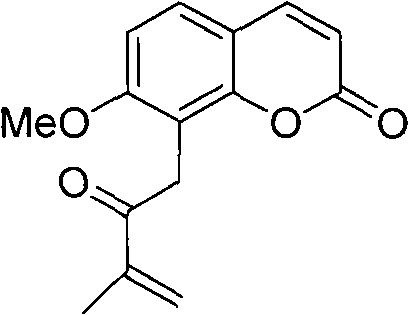
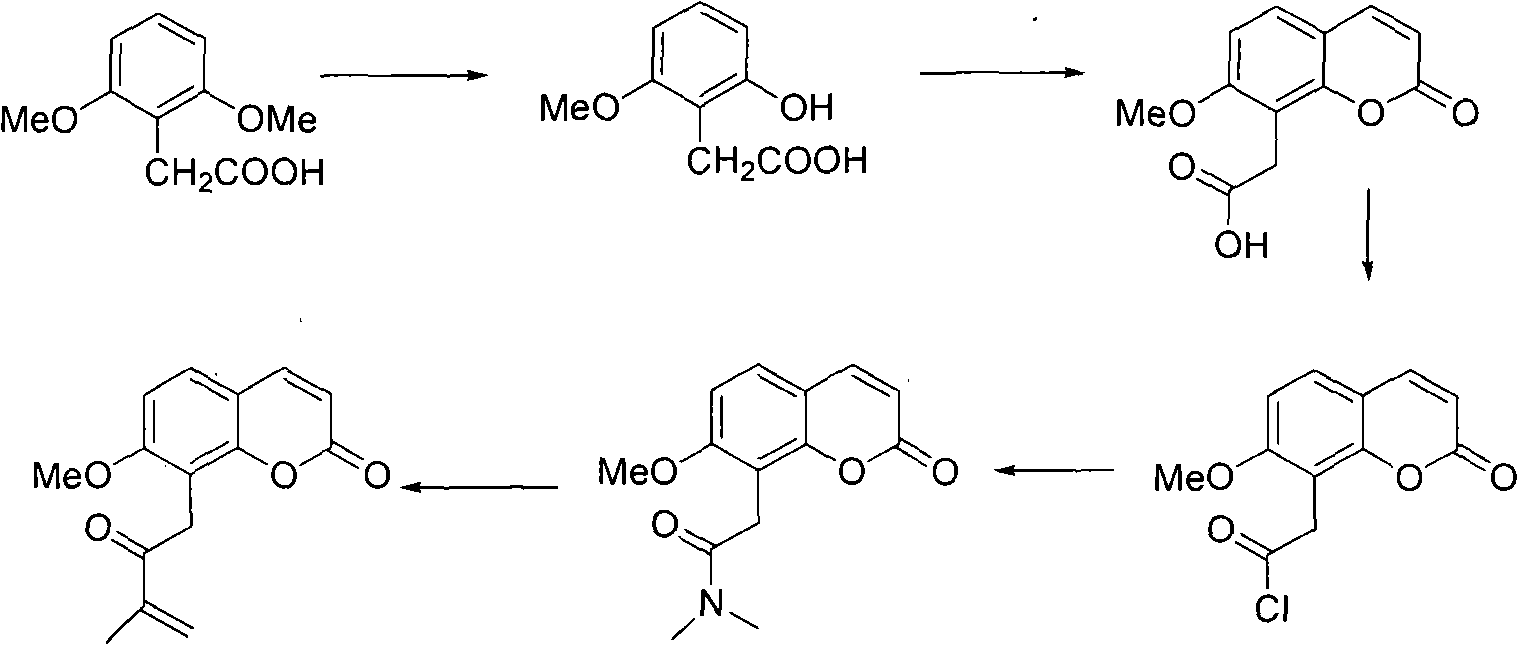
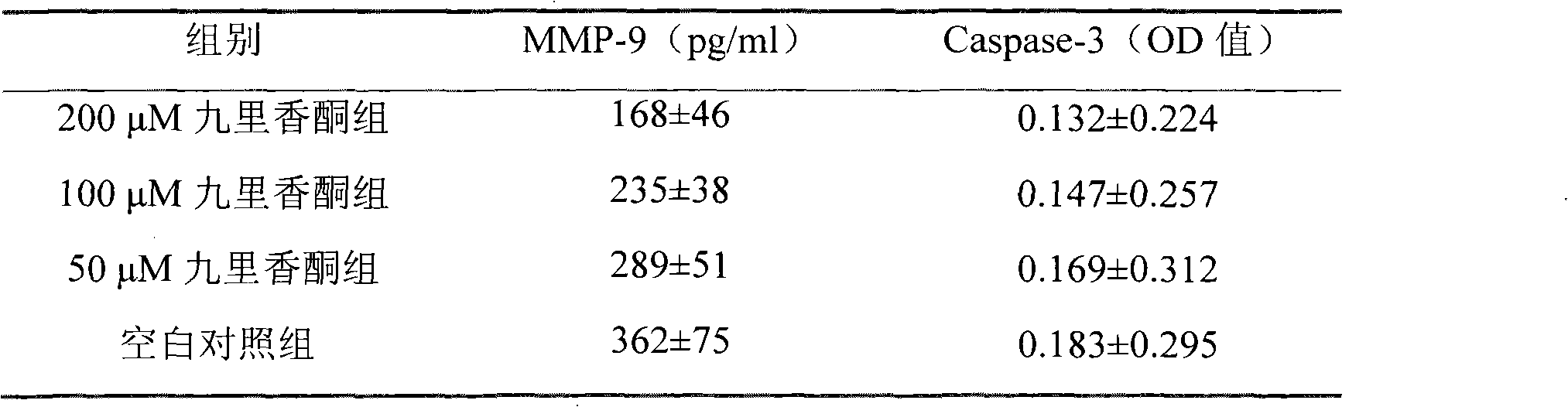
Novel synthesis method of murrayone and novel application of murrayone
A synthesis method and a technique for jurisone, applied in the field of medicine, can solve the problems of complex process, difficult operation, harsh conditions and the like, and achieve the effects of simple operation, easy availability of raw materials and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1 9
[0021] Embodiment 1 Jiu Lixiang ketone synthetic preparation-I
[0022] Step A: Dissolve 19.6 g (0.1 mol) of 2,6-dimethoxyphenylacetic acid in 200 ml of anhydrous CH 2 Cl 2 10.9ml (0.115mol) BBr was added dropwise under stirring at -30°C 3 , after the dropwise addition, the reaction temperature was raised to room temperature, stirred overnight, and quenched by adding ice water after 10 hours of reaction, and the water phase was decomposed with CH 2 Cl 2 Extract three times, combine the organic phases, distill off the CH 2 Cl 2 , 11.4 g of 2-hydroxy-6-methoxyphenylacetic acid could be obtained by column chromatography (yield: 62.8%). used directly in the next reaction.
[0023] Step B: Mix 9.1g (0.05mol) of 2-hydroxy-6-methoxyphenylacetic acid with 17.5ml (0.175mol) of ethyl propiolate, add 6.8g of anhydrous aluminum trichloride under stirring, and heat To 100°C, react for 2h, add ice water to quench, stir and filter. The filter cake was dissolved with ethyl acetate, wa...
Embodiment 2 9
[0026] Example 2 Synthetic Preparation of Jiulixiang Ketone-II
[0027] Step A: Dissolve 19.6 g (0.1 mol) of 2,6-dimethoxyphenylacetic acid in 200 ml of anhydrous CH 2 Cl 2 Add 9.3ml (0.1mol) BBr dropwise under stirring at -35°C 3 , after the dropwise addition, the reaction temperature was raised to room temperature, stirred overnight, and quenched by adding ice water after 11 hours of reaction, and the water phase was decomposed with CH 2 Cl 2 Extract three times, combine the organic phases, distill off the CH 2 Cl 2 , 9.7 g of 2-hydroxy-6-methoxyphenylacetic acid could be obtained by column chromatography (yield: 53.6%). used directly in the next reaction.
[0028] Step B: Mix 9.1g (0.05mol) of 2-hydroxy-6-methoxyphenylacetic acid with 10.0ml (0.1mol) ethyl propiolate, add 6.8g of anhydrous tin tetrachloride under stirring, and heat To 95°C, react for 1.5h, add ice water to quench, stir and filter. The filter cake was dissolved in ethyl acetate, washed with 1mol / L HC...
Embodiment 3 9
[0031] Example 3 Synthetic Preparation of Jiulixiang Ketone-III
[0032] Step A: Dissolve 19.6 g (0.1 mol) of 2,6-dimethoxyphenylacetic acid in 200 ml of anhydrous CH 2 Cl 2 9.3ml (0.1mol) BBr was added dropwise under stirring at -30°C 3 , after the dropwise addition, the reaction temperature was raised to room temperature, stirred overnight, and quenched by adding ice water after 10 hours of reaction, and the water phase was decomposed with CH 2 Cl 2 Extract three times, combine the organic phases, distill off the CH 2 Cl 2 , 10.8 g (yield: 59.5%) of 2-hydroxy-6-methoxyphenylacetic acid could be obtained by column chromatography. used directly in the next reaction.
[0033] Step B: Mix 9.1g (0.05mol) of 2-hydroxy-6-methoxyphenylacetic acid with 17.5ml (0.175mol) of ethyl propiolate, add 6.8g of anhydrous zinc chloride under stirring, and heat to 100°C, react for 2h, add ice water to quench, stir and filter. The filter cake was dissolved in ethyl acetate, washed with 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com