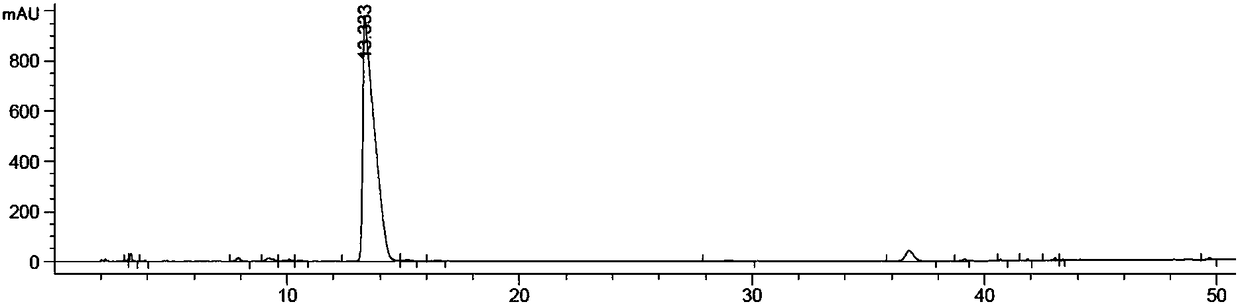
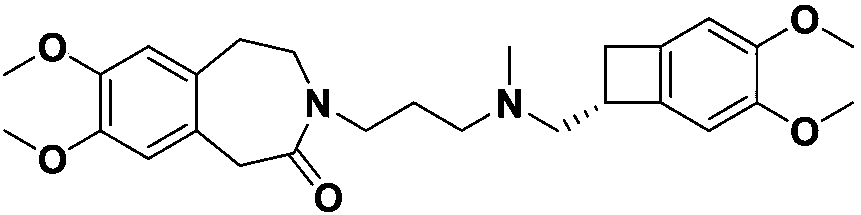
Preparation method for ivabradine impurities
An ivabradine and impurity technology is applied in the field of preparation of ivabradine impurities, and achieves the effects of high purity, improved drug safety and simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Embodiment 1: the preparation of impurity A
[0046] 15g of the compound of formula 1a, 65g of potassium carbonate and 300ml of acetone were mixed and stirred. Add 41.5 g of benzyl bromide dropwise to the system at a uniform speed, and after the addition is completed, the temperature is raised to reflux for overnight reaction. After the reaction was completed, it was lowered to room temperature, filtered, and the filtrate was collected and concentrated under reduced pressure to obtain 37 g of the compound of formula 2, which was directly injected into the next step without purification.
[0047] 37g of the compound of formula 2 and 180ml of ethanol were mixed and stirred. Add 160ml of aqueous solution mixed with 6.3g of sodium hydroxide at a uniform speed, and after the addition is complete, raise the temperature to 50°C for reaction. After the reaction, cool to room temperature. Most of the ethanol was distilled off under reduced pressure, 200ml of ethyl acetate and...
Embodiment 2
[0054] The synthesis of embodiment 2 impurity B
[0055] 30g of the compound of formula 1b, 120g of sodium carbonate and 600ml of toluene were mixed and stirred. Add 82g of benzyl chloride dropwise to the system at a constant speed, and after the addition, raise the temperature to 60°C to react overnight. After the reaction was completed, it was lowered to room temperature, filtered, and the filtrate was collected and concentrated under reduced pressure to obtain 50 g of the compound of formula 2, which was directly injected into the next step without purification.
[0056] 30g of the compound of formula 2 and 180ml of ethanol were mixed and stirred. Add 100ml of aqueous solution mixed with 6.3g of potassium hydroxide at a constant speed, and after the addition is complete, rise to 45°C for reaction. After the reaction, cool to room temperature. Most of the ethanol was distilled off under reduced pressure, 200ml of ethyl acetate and 150ml of water were added for extraction,...
Embodiment 3
[0063] Embodiment 3: the preparation of impurity A
[0064] 5g of the compound of formula 1a and 3.75g of 2,2-dimethoxyethylamine were mixed and dissolved in 50ml of dichloromethane, and stirred evenly. Then drop into 6.3g 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDCI), 4.4g 1-hydroxybenzotriazole (HOBT) successively, after adding, room temperature reaction. After the reaction, the reaction system was added to 100ml of saturated aqueous sodium bicarbonate solution for extraction, and the aqueous layer was back-extracted once with 100ml of dichloromethane, the dichloromethane layer was collected, dried over anhydrous sodium sulfate, filtered, and concentrated to obtain 5.8g of the compound of formula 2a . No purification required, directly submitted to the next step.
[0065] In a 100ml one-necked flask, add 5g of the compound of formula 2a, 10ml of glacial acetic acid and 3ml of concentrated hydrochloric acid, and react overnight at room temperature afte...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com