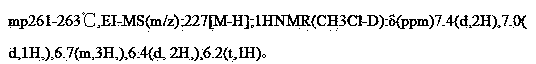Preparation method for resveratrol
A technology of resveratrol and methoxybenzaldehyde dimethyl acetal is applied in the preparation of organic compounds, chemical instruments and methods, organic chemistry and other directions, and can solve the problems of long synthesis lines, high production costs, environmental pollution and the like, Achieve the effect of good quality, low preparation cost and short process flow
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
reference example 1
[0034] Reference example 1: the preparation of raw material p-methoxybenzaldehyde dimethyl acetal:
[0035] In a 500ml three-neck flask equipped with a thermometer and stirring, carefully add 30 grams of p-methoxybenzaldehyde and 210ml of anhydrous methanol, cool to 0°C, replace with nitrogen three times, start stirring, and carefully pass in dry bromine About 20g of hydrogen hydride gas was allowed to stabilize for 1 hour. After the reaction of the raw material was detected by TLC, it was concentrated under reduced pressure to constant weight to obtain 33g of yellow oil, yield: 99%, and stored at low temperature for future use.
[0036] EI-MS(m / z):182[M+H];1HNMR(CH3Cl-D):δ(ppm)7.1(d,2H),5.5(d,1H,),6.7(m,2H,), 3.4(d, 6H,), 3.7(s, 3H).
Embodiment 1
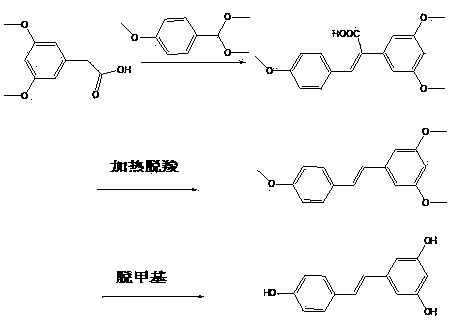
[0038]In a 500ml three-necked flask equipped with a thermometer and a stirrer, 40g of 3,5-dimethoxyphenylacetic acid and 200ml of toluene were added to 43g of p-methoxybenzaldehyde dimethyl acetal in the reaction flask, and 10mL was added dropwise. Acetic anhydride, after the dropwise addition, add 3g triethylamine, reflux for 2 hours; after the reaction of the raw materials is detected by TLC, add 20ml quinoline and 5g copper powder, heat and reflux for 12 hours, after the plate decarboxylation is completed, add 40g of triethylamine in 5 batches Anhydrous aluminum chloride, when adding, control the temperature below 60°C. After the addition, raise the temperature to 70°C, and react for 10 hours. After the reaction of the raw materials is detected by TLC, the developing agent: EA / PE=2:1, carefully added to 2L ice water, the temperature does not exceed 40°C when adding, filter, separate toluene layer, adjust the pH of the water layer to 1-2 with concentrated hydrochloric acid, e...
reference example 2
[0042] Reference example 2 The preparation of raw material p-methoxybenzaldehyde ethylene acetal:
[0043] In a 500ml three-necked flask equipped with a thermometer and stirring, carefully add 30 grams of raw material p-methoxybenzaldehyde and 210ml of anhydrous ethylene glycol, after cooling to 0°C, replace with nitrogen three times, start stirring, and carefully pass through to dry About 20g of hydrogen bromide gas was allowed to stabilize for 1 hour. After the reaction of the raw material was detected by TLC, it was directly passed through the column. The eluent was PE / EA=3:1. After concentration, 28g of yellow oil was obtained. Yield: 92% , stored at low temperature for later use.
[0044] EI-MS(m / z):180[M+H];1HNMR(CH3Cl-D):δ(ppm)7.1(d,2H),5.5(d,1H,),6.7(m,2H,), 3.9(d, 4H,), 3.7(s, 3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com