Synthesis method of elagolix intermediate
A synthetic method, the technology of Eragoli, which is applied in the field of medicine and chemical industry, can solve the problems of low industrialization value, low atomic economy, and high cost, and achieve high utilization of atomic economy, easy availability of raw materials, and reduced process costs Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
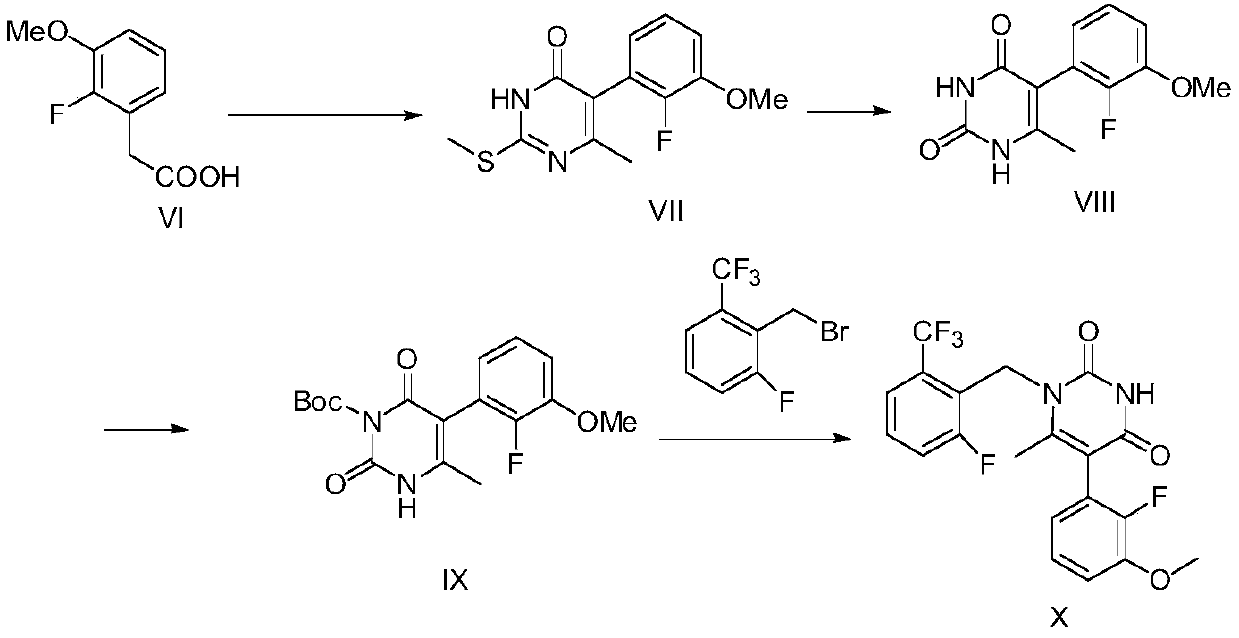
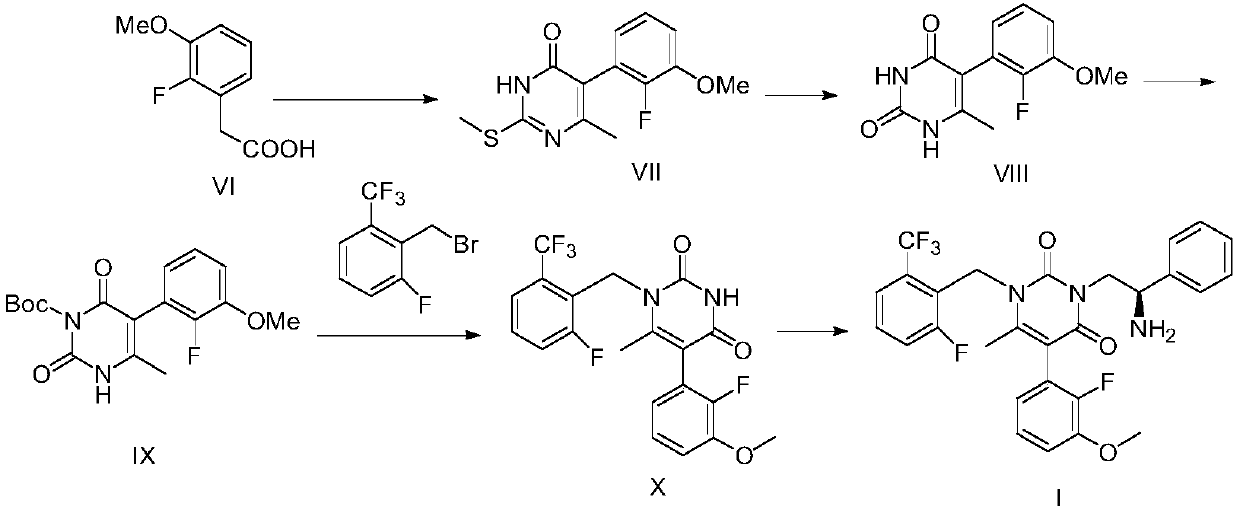
[0077] The present invention provides a kind of synthetic method of elagolix intermediate compound X, and described method comprises steps:
[0078]
[0079] (a) in an inert solvent, compound VI is subjected to a cyclization reaction with 1-[1-(dimethylamino)ethylene]-2-methylthiourea salt to form compound VII;
[0080] (b) subjecting compound VII to a hydrolysis reaction to form compound VIII;
[0081] (c) in an inert solvent, compound VIII is subjected to an amino-protection reaction with an amino-protecting reagent, thereby forming compound IX;
[0082] (d) first in an inert solvent, in the presence of a base, compound IX and 2-trifluoromethyl-6-fluoro-benzyl bromide are subjected to a condensation reaction; after the condensation reaction is completed, the mixture containing the condensation product is subjected to acidic conditions The amino group deprotection reaction is carried out, thereby forming the elagolix intermediate compound X.
[0083] The present inventio...
Embodiment 1
[0121] Add 2-fluoroanisole (85.0 g, 0.67 mol), tetrahydrofuran (3540 mL), tetramethylethylenediamine (78.3 g, 0.67 mol) into a three-necked flask at room temperature, replace with nitrogen three times, start stirring, and cool down to -50 ~-78°C, add sec-butyllithium solution (870mL, 1.3M) dropwise, keep stirring for 2-3 hours after the dropwise addition, add N,N-dimethylformamide (67.5g, 0.92mol) dropwise, keep warm Stir for 1 hour, after the reaction is completed, add 13% acetic acid aqueous solution (1464g) dropwise at -50~-78°C, separate the layers, extract the aqueous phase with ethyl acetate (350mL*3), combine the organic phases, wash with water (500mL) and then 1N After washing with hydrochloric acid, the organic phase was concentrated until there was no liquid drop to obtain a light yellow mixture, which was crystallized from methyl tert-butyl ether (150 mL) to obtain a white solid, which was dried to obtain compound II (57.7 g, 57.3%). 1 HNMR-(300MHz, CDCl 3 )δ, 10.3...
Embodiment 2
[0123] Add compound II (104.8g, 0.68mol) and sodium borohydride (13.0g, 0.34mol) to a three-necked flask at room temperature, add methanol (1000mL), and stir at 15-50°C for 0.5-1.5 hours. After the reaction, 5% dilute hydrochloric acid aqueous solution was added dropwise to the system, extracted with ethyl acetate, separated, the organic phase was washed with saturated brine, and the organic phase was concentrated until no liquid was evaporated to obtain compound III (107.0 g, 100%). 1 HNMR-(300MHz, CDCl 3 )δ: 7.05-7.07 (m, 1H), 6.99-7.01 (s, 1H), 6.92-6.94 (m, 1H), 4.76 (s, 2H), 3.89 (s, 3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com