Synthesis technology of higenamine and pharmaceutical salt of higenamine
A technology of higenamine base and synthesis process, which is applied in the direction of organic chemistry, can solve the problems of little industrial application value, high cost of raw materials, high risk, etc., and achieve easy control of the reaction process, low post-processing cost, The effect of low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
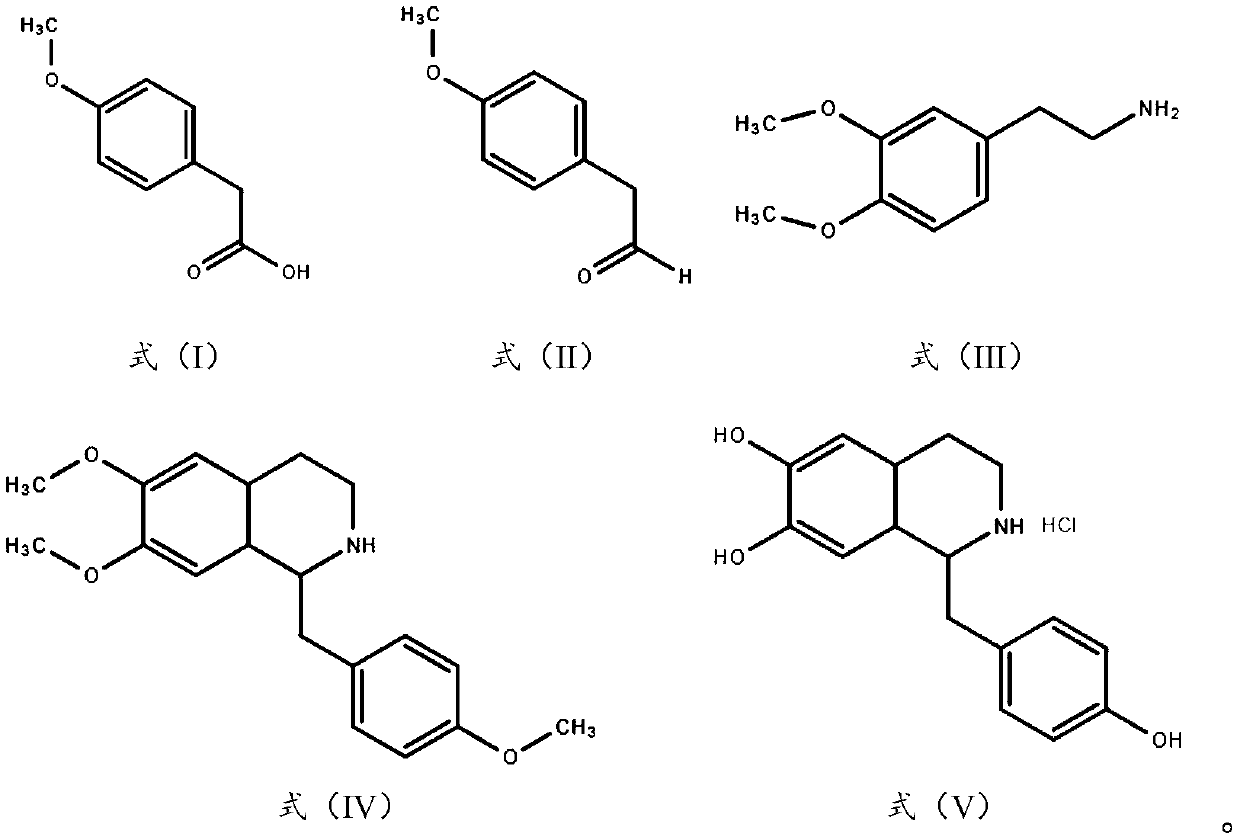
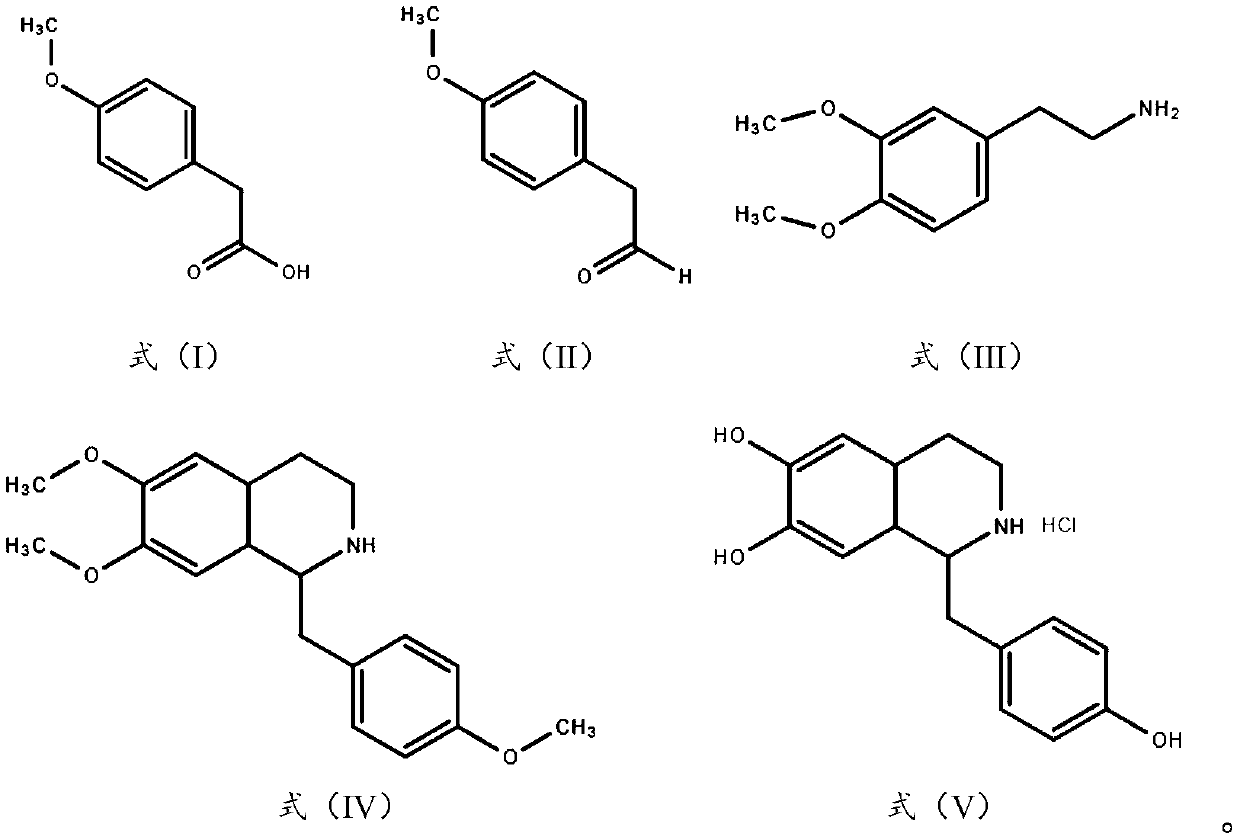
[0028] (1) Preparation of 4-methoxyphenylacetaldehyde
[0029] Dissolve 24.07g of sodium hydroxide in 500g of water, add 100g of 4-methoxyphenylacetic acid, stir for 30min to completely dissolve the solid, add 38.95g of potassium borohydride and 8.20g of zinc chloride, raise the temperature to 45°C for 3h, and use After TLC monitors that 4-methoxyphenylacetic acid is consumed, 15% hydrochloric acid is added dropwise to neutralize the reaction solution to pH=7, 62.78g of active manganese dioxide is added, and the reaction is continued for 4h. After the reaction is complete, the reaction solution is cooled to room temperature, and 1mol / L sodium hydroxide solution to adjust the pH to 8-9, add 500g of dichloromethane and stir for 1 hour, let stand for liquid separation, take the organic phase, dry it with anhydrous sodium sulfate, and filter to obtain 4-methoxyphenylacetaldehyde solution.
[0030] (2) Preparation of 6,7-dimethoxy-1-(4-methoxybenzyl)-1,2,3,4-tetrahydroisoquinoline ...
Embodiment 2
[0035] (1) Preparation of 4-methoxyphenylacetaldehyde
[0036] Dissolve 41.59g of potassium carbonate in 600g of dichloromethane, add 100g of 4-methoxyphenylacetic acid, stir for 40min to completely dissolve the solid, add 22.77g of sodium borohydride and 7.64g of iodine, heat up to 45°C for 2h, and use After TLC monitors that 4-methoxyphenylacetic acid is consumed, 10% hydrochloric acid is added dropwise to neutralize the reaction solution to pH=7, 78.48g active manganese dioxide is added, and the reaction is continued for 3h. After the reaction is complete, the reaction solution is cooled to room temperature, and 1mol / L sodium hydroxide solution to adjust the pH to 8-9, add 600g of water and stir for 1 hour, let stand for liquid separation, take the organic phase and dry it with anhydrous sodium sulfate, filter to obtain 4-methoxyphenylacetaldehyde solution.
[0037] (2) Preparation of 6,7-dimethoxy-1-(4-methoxybenzyl)-1,2,3,4-tetrahydroisoquinoline
[0038] Add 109.06g of ...
Embodiment 3
[0042] (1) Preparation of 4-methoxyphenylacetaldehyde
[0043] Dissolve 41.59g of sodium bicarbonate in 400g of tetrahydrofuran, add 100g of 4-methoxyphenylacetic acid, stir for 60 minutes to completely dissolve the solid, add 103.13g of sodium thioborohydride and 4.04g of copper chloride, and heat up to 50°C for reaction 3h, after the consumption of 4-methoxyphenylacetic acid was monitored by TLC, 10% hydrochloric acid was added dropwise to neutralize the reaction solution to pH=7, 94.17g active manganese dioxide was added, and the reaction was continued for 5h. After the reaction was complete, the reaction solution was cooled to At room temperature, use 1 mol / L sodium hydroxide solution to adjust the pH to 8-9, add 400 g of water and stir for 1 h, let stand to separate the liquids, take the organic phase, dry it with anhydrous sodium sulfate, and filter to obtain a 4-methoxyphenylacetaldehyde solution.
[0044] (2) Preparation of 6,7-dimethoxy-1-(4-methoxybenzyl)-1,2,3,4-tet...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com