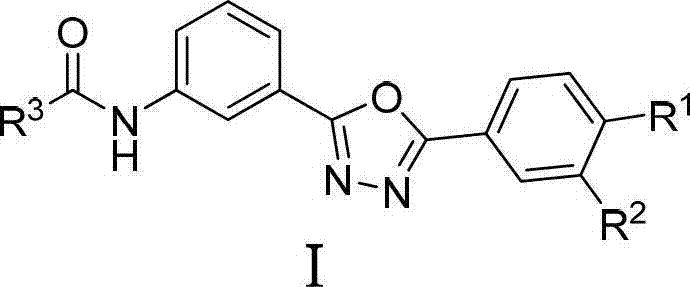
2,5-diaryl-1,3,4-oxadiazole compounds and preparation method and application thereof
A technology for oxadiazoles and compounds, which is applied in the fields of drug combination, organic chemistry, medical preparations containing active ingredients, etc., can solve the problems of limited types of compounds, poor activity and bioavailability, and reduce the risk of side effects Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
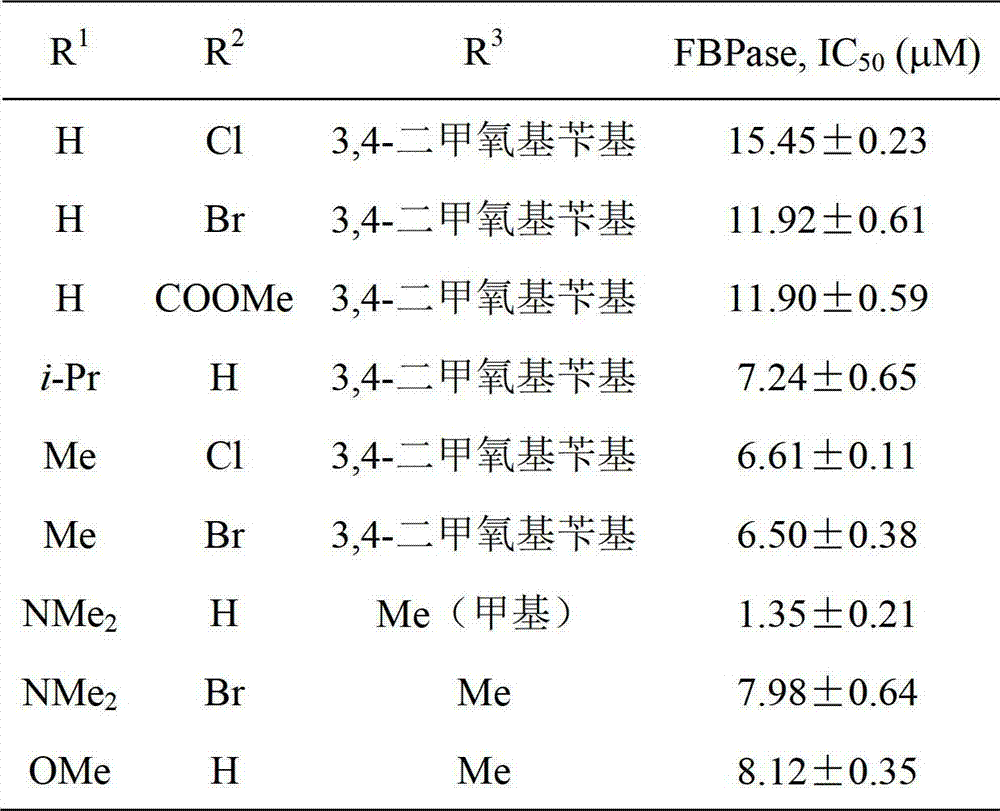
[0035] Compound I (R 1 =H, R 2 =Cl, R 3 =3,4-Dimethoxybenzyl) Synthesis:
[0036] (A) m-nitrobenzoyl hydrazide (1.3g, 7.3mmol), m-chlorobenzoic acid (1.14g, 7.3mmol), EDC (1.8g, 9.5mmol) and HOBt (1.42g, 9.5mmol) are suspended in two After stirring for 10 min in methyl chloride (20 mL), pyridine (1.5 mL) was added to the above reaction solution, and after stirring and reacting for 18 h, a large amount of solid was precipitated. Add 30 mL of 10% dilute hydrochloric acid to the reaction solution, stir for 30 min, filter, and the filter cake is washed thoroughly with water and dried to obtain an off-white solid i (R 1 =H, R 2 =Cl) 1.55g, yield: 67%.
[0037] (B) The product i(R 1 =H, R 2 =Cl, 3.19g, 10mmol) was suspended in acetonitrile (45mL), followed by phosphorus oxychloride (2.32mL). The above mixture was heated to 75°C to react for 10h, cooled to room temperature and concentrated under reduced pressure to near dryness. Under ice-water bath cooling, add 120 mL each of a dichlor...
Embodiment 2
[0041] Compound I (R 1 =NMe 2 , R 2 =H, R 3 =Me) synthesis:
[0042] (A) m-nitrobenzoyl hydrazide (1.3g, 7.3mmol), 4-N,N-dimethylbenzoic acid (1.2g, 7.3mmol), EDC (2.77g, 14.5mmol) and HOBt (2.2, 14.5mmol) was suspended in dry THF (20mL). After stirring for 10min, 3-methylpyridine (1.5mL) was added to the above reaction solution. After stirring and reacting for 36h, a large amount of solid was precipitated. After filtration, the filter cake is fully washed with water and then dried to obtain an off-white solid. The product i(R 1 =NMe 2 , R 2 =H) 1.35g, yield: 56%.
[0043] (B) Suspend the product i (1.2 g, 3.66 mmol) obtained in the previous step in acetonitrile (15 mL), and then add phosphorus oxychloride (0.51 mL). The above mixture was heated to reflux for 8h, cooled to room temperature and concentrated under reduced pressure to near dryness. Under ice-water bath cooling, add 40 mL each of a dichloromethane / methanol (5 / 1) mixed solution and 5% NaOH solution to the above concen...
Embodiment 3
[0047] Compound I (R 1 =Me, R 2 =Cl, R 3 =3,4-Dimethoxybenzyl) Synthesis:
[0048] Using 4-methyl-3-chlorobenzoic acid as a raw material, it was synthesized by referring to the method in Example 1. The total yield of the four-step reaction was 27.8%. White solid 1 H NMR(500MHz, CDCl 3 ): δ2.41(s,3H), 3.72(s,2H), 3.84(s,3H), 3.85(s,3H), 6.83-6.88(m,3H), 7.32(d,J=7.5Hz, 1H),7.40(t,J=8.0Hz,1H),7.77-7.79(m,2H),7.83(dd,J 1 =1.5Hz, J 2 =7.9Hz,1H),8.01(d,J=1.4Hz,1H),8.07(s,1H),8.18(s,1H); 13 C NMR(125MHz, CDCl 3 ): δ170.0, 164.2, 163.6, 149.3, 148.5, 140.3, 138.7, 135.1, 131.5, 129.7, 127.2, 126.6, 124.9, 124.1, 123.2, 122.6 (2C), 121.6, 117.9, 112.5, 111.6, 55.8 (2C), 44.1,20.2;HRMS(ESI):Calcd for C 25 H 23 ClN 3 O 4 [M+H] + , 464.1377; Found, 464.1377.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com