Vaccine for preventing novel coronavirus pneumonia COVID-19 and preparation method thereof
A technology of CRM197, SEQIDNO.1-SEQIDNO.11, which is applied in the field of vaccines and preparations for the prevention of novel coronavirus pneumonia COVID-19, can solve the problems such as the loss of efficacy of biohazardous original vaccines, and reduce research and development costs and research and development cycles , enhance the immune response of the human body, and facilitate large-scale mass production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Example 1 Synthetic method of target polypeptide.
[0061] 1. The polypeptide whose amino acid sequence is shown in SEQ ID NO.1-SEQNO.11 was prepared by conventional solid-phase polypeptide synthesis method, and the specific steps are as follows.
[0062] (1) Remove Fmoc protection.
[0063] Fill a commercially available Rink Amide AM resin into an organic-solvent-resistant reaction tube with a filter and cap tightly with an organic-solvent-resistant cap. After washing with DMF (dimethylformamide) for 1 minute, add an excess of 20% piperidine / DMF (volume ratio) solution, cap tightly and shake the reaction tube gently to mix it evenly and keep the Fmoc protection removed for 15 minutes . After draining, wash three times with DMF.
[0064] (2) Peptide bond condensation.
[0065] The Fmoc-protected amino acid of 3 times the molar weight of the resin amino group, the activator HBTU of 3 times the molar equivalent of the resin amino group, and the DIPEA (N , N-diisoprop...
Embodiment 2
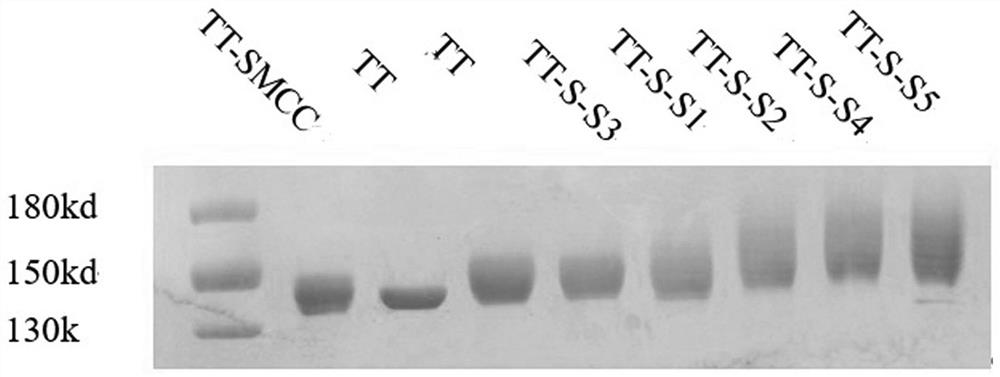
[0077]Example 2 Preparation method of immunogenic conjugate.
[0078] The list of conjugates formed by the synthetic peptides (S1-S11) obtained in Example 1 with CRM197, TT, DT, and M-M-OMP respectively through SMCC LINKER or DSAP LINKER is shown in Table 2.
[0079] TT tetanus toxin protein and tetanus toxin can be obtained from Bacillus tetani through fermentation, lysis, centrifugation and chromatographic purification.
[0080] DT diphtheria toxin protein and diphtheria toxin carrier protein DT can be obtained from diphtheria bacillus through fermentation, cracking, centrifugation and chromatography purification.
[0081] CRM197 Recombinant Diphtheria Toxin Protein, CRM197 is a diphtheria bacillus reconstructed from the gene sequence, which is obtained through fermentation, lysis, centrifugation, and chromatography purification.
[0082] Meningococcal outer membrane protein (M-OMP), obtained from meningococcus, is fermented, lysed, centrifuged, and chromatographically puri...
Embodiment 3 4
[0098] Example 3 Preparation and immune effect of tetravalent peptide vaccine.
[0099] 1. Vaccine preparation.
[0100] 1. Materials.
[0101] The polypeptide conjugate prepared in Example 2.
[0102] Adjuvants: aluminum hydroxide (Al(OH)3), aluminum phosphate (AlPO4), MPL, CpG.
[0103] 2. Preparation method.
[0104] Select the immunogenic conjugates combined with the synthetic peptides in Example 2 and different carrier proteins, preferably different immunogenic conjugates with sequences SEQID NO.4, 5, 6, and 7, and combine them with different adjuvants to prepare four For peptide vaccines, the total concentration of the four peptides is 100ug / ml, and the specific combination scheme is shown in Table 3.
[0105] Table 3. Cross-combination scheme of polypeptide conjugates and adjuvants.
[0106]
[0107] Note: Combination serial number naming rules: arrange the conjugates according to the size of the number, "conjugate carrier initial letter" + "adjuvant number".
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com