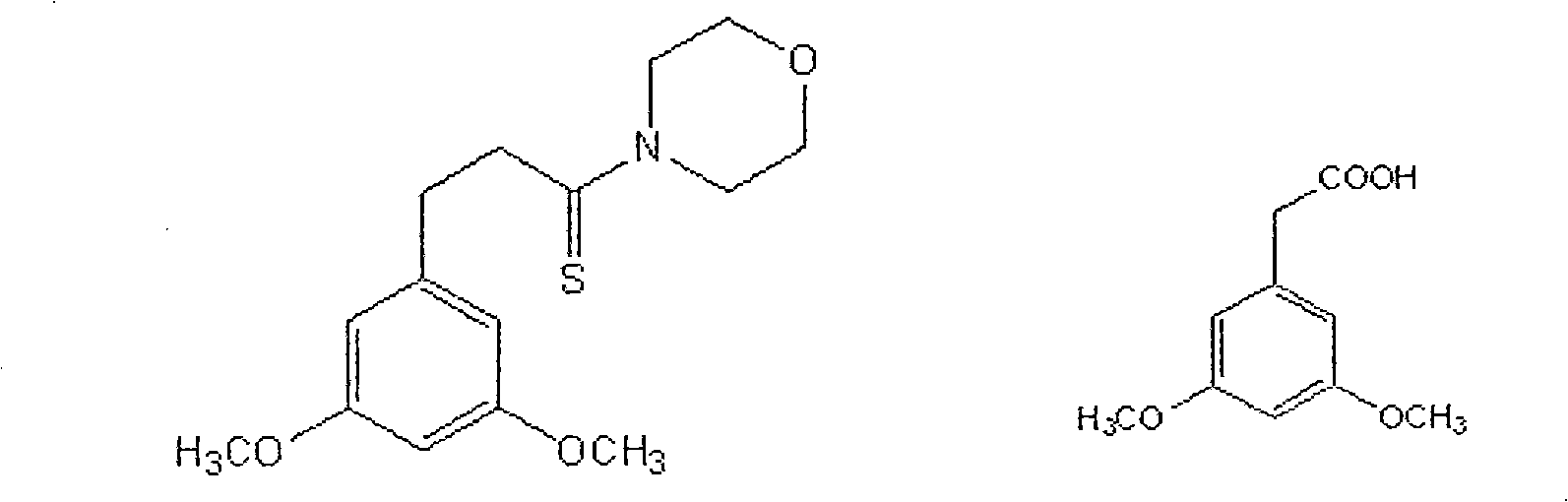
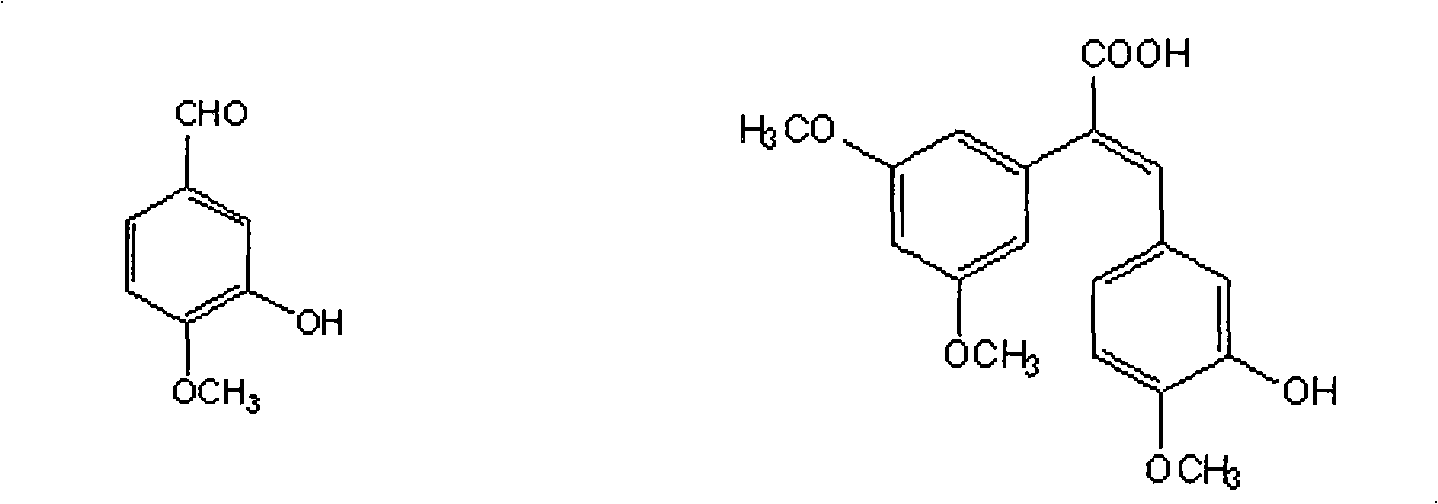
Method for preparing (Z)-3'-hydroxy-3,4',5-trimethoxy diphenylethene
A technology of trimethoxystilbene and dimethoxyacetophenone, which is applied in the field of -3'-hydroxyl-3, can solve the problem of long reaction time in the step of bromine-to-hydroxyl, difficulty in realizing large-scale preparation, and cis-selectivity. low problems, to achieve the effect of low price, low cost and high cis-selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040]1.52g (0.010mol) of 3,5-dihydroxyacetophenone and 10ml of water were added to the reaction flask, and 1.2g (0.030mol) of sodium hydroxide (prepared as an aqueous solution, The concentration is about 20% by mass) and 2.36ml (0.025mol) of dimethyl sulfate solution. After about 1.5h of dropwise addition, continue to stir and react for 1h. 1.73 g of solid 3,5-dimethoxyacetophenone, yield 96.11%.
Embodiment 2
[0042] Add 1.52g (0.010mol) of 3,5-dihydroxyacetophenone and 10ml of water into the reaction flask, and add 0.80g (0.020mol) of about 20% by mass of hydrogen hydroxide dropwise while stirring in an ice-water bath (0°C). Sodium aqueous solution and 1.89ml (0.020mol) of dimethyl sulfate solution, about 1h after the dropwise addition, continue to stir and react for 1.5h, pour the reaction solution into 20ml of ice water and let it stand for cooling, and suction filter to obtain a brown solid 3,5- Dimethoxyacetophenone 1.21g, yield 67.22%.
Embodiment 3
[0044] 1.52g (0.010mol) of 3,5-dihydroxyacetophenone and 10ml of water were added to the reaction flask, and 2.40g (0.060mol) of about 20% by mass aqueous sodium hydroxide solution and 4.72ml (0.050mol) of dimethyl sulfate solution, about 3h after the dropwise addition, continue to stir the reaction for 6h, pour the reaction solution into 20ml of ice water and let it cool down, then suction filter to obtain brown solid 3,5-dimethoxy Acetophenone 1.37g, yield 76.11%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com