Preparation method of chiral higenamine and derivatives of chiral higenamine
A technology of higenamine and chirality, which is applied in the field of preparation of chiral higenamine and its derivatives, and can solve the problem that it is difficult to meet the medical use requirements of higenamine isomers, and it is difficult to obtain higenamine Eliminate problems such as isomers of hiratine, and achieve high selectivity and high product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Embodiment one: the preparation of (R)-higenamine hydrochloride
[0041] Take a 500mL three-neck flask, add 40mL dichloromethane and 20g (0.12mol) 4-methoxyphenylacetic acid (compound III) under nitrogen protection, start stirring, cool to 0°C in an ice-salt bath, add 1.75g (0.024mol) ) N,N-dimethylformamide as catalyst, then slowly add 18.3g (0.144mol) oxalyl chloride dropwise, raise the temperature to 25°C, and stir for 4 hours. When TCL detects that the reaction of compound III is complete, stop stirring, and distill under reduced pressure The solvent was removed to obtain 23.8 g of 4-methoxyphenylacetyl chloride (compound IV) as an oil.
[0042] Dissolve 21.7g (0.12mol) of 2-(3,4-dimethoxy)phenethylamine in 40mL of dichloromethane, add 12.1g (0.12mol) of triethylamine as an acid trap, and cool in an ice-salt bath to 0°C, dissolve 23.8g of the obtained oily 4-methoxyphenylacetyl chloride (compound IV) in 40mL of dry dichloromethane and slowly add it dropwise to th...
Embodiment 2
[0055] Embodiment two: the preparation of (S)-higenamine hydrobromide
[0056]Take a 500mL three-necked flask, add 40mL dichloromethane and 20g (0.12mol) 4-methoxyphenylacetic acid (compound III) under nitrogen protection, start stirring, cool to 0°C in an ice-salt bath, add 4.38g (0.06mol) ) N,N-dimethylformamide as a catalyst, then slowly add 28.6g (0.24mol) of thionyl chloride dropwise, raise the temperature to 45°C, stir and reflux for 3 hours, when TCL detects that the reaction of compound III is complete, stop stirring, The solvent was distilled off under reduced pressure to obtain 22.3 g of oily 4-methoxyphenylacetyl chloride (compound IV).
[0057] Dissolve 21g (0.116mol) of 2-(3,4-dimethoxy)phenethylamine in 35mL of dichloromethane, add 9.2g (0.116mol) of pyridine as an acid trap, cool to 0-5°C, and The prepared 22.3g of oily 4-methoxyphenylacetyl chloride (compound IV) was dissolved in 45mL of dry dichloromethane and slowly added dropwise to the reaction solution. ...
Embodiment 3
[0070] Embodiment three: structural identification
[0071] Adopt elemental analyzer, measure respectively the C, H, H, N composition, the measurement results are shown in Table 1 and Table 2.
[0072] Table 1 (R) - elemental analysis results of higenamine hydrochloride
[0073]
[0074] Table 2 (S) - elemental analysis results of higenamine hydrobromide
[0075]
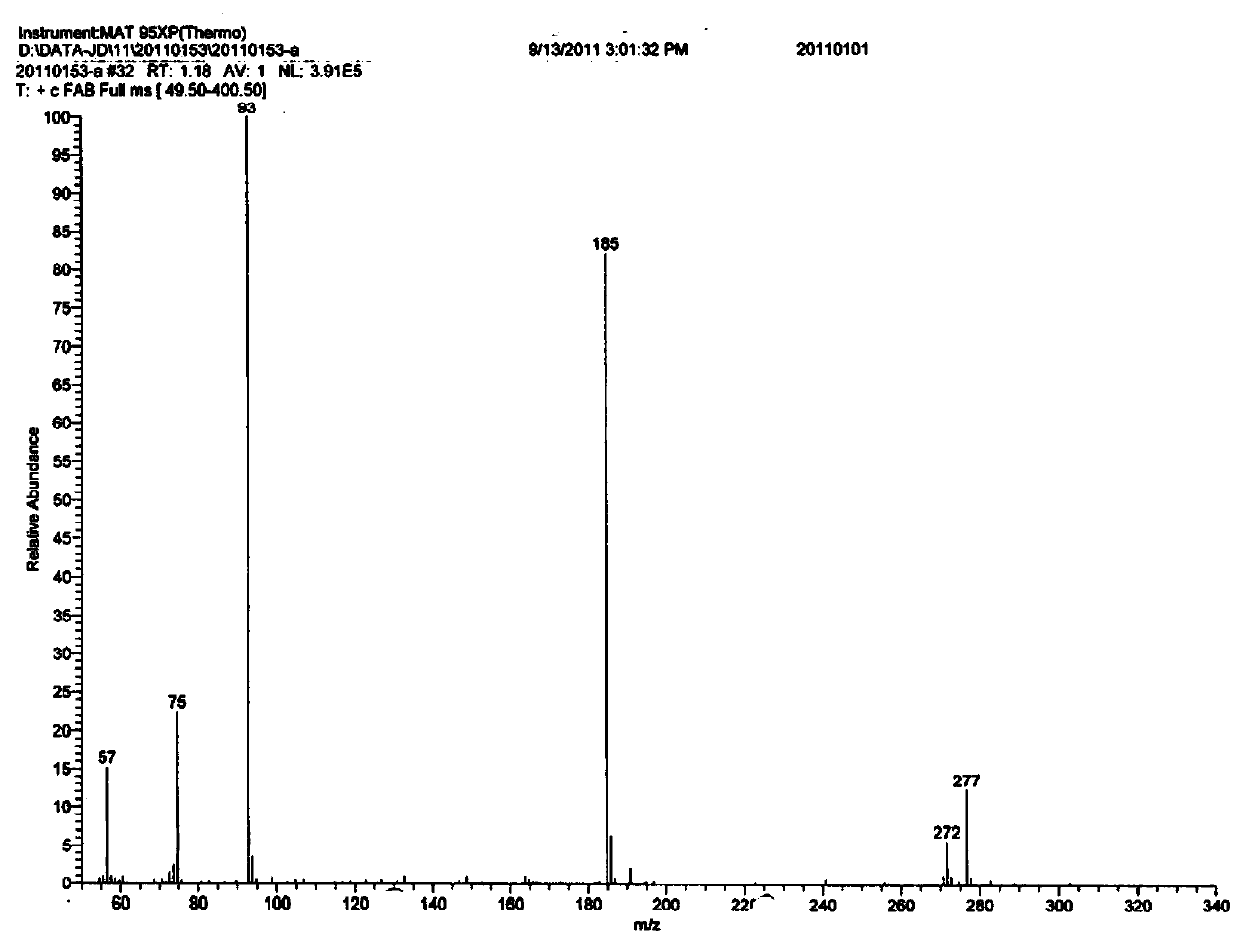
[0076] Using a high-resolution mass spectrometer (Thermo Finnigan of U.S. Thermo Finnigan, the model is MAT95XP), the (R)-higenamine hydrochloride prepared in Example 1 and the ( S)-higenamine hydrobromide was subjected to mass spectrometry analysis, and its fast atom bombardment ionization (+FAB) mass spectrometry results were as follows figure 1 , figure 2 shown. figure 1 , figure 2 The assignment of the m / z272 ion peak in is as follows:
[0077]
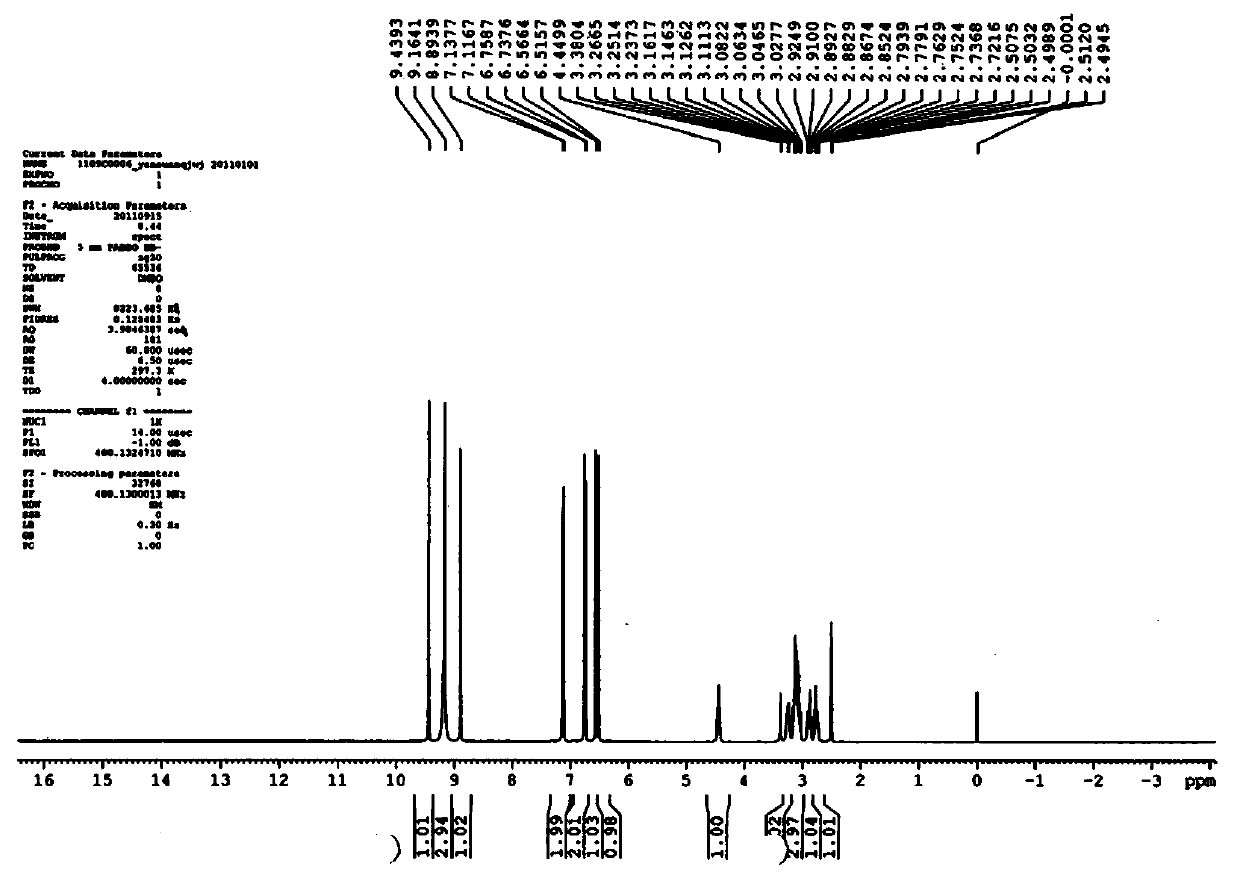
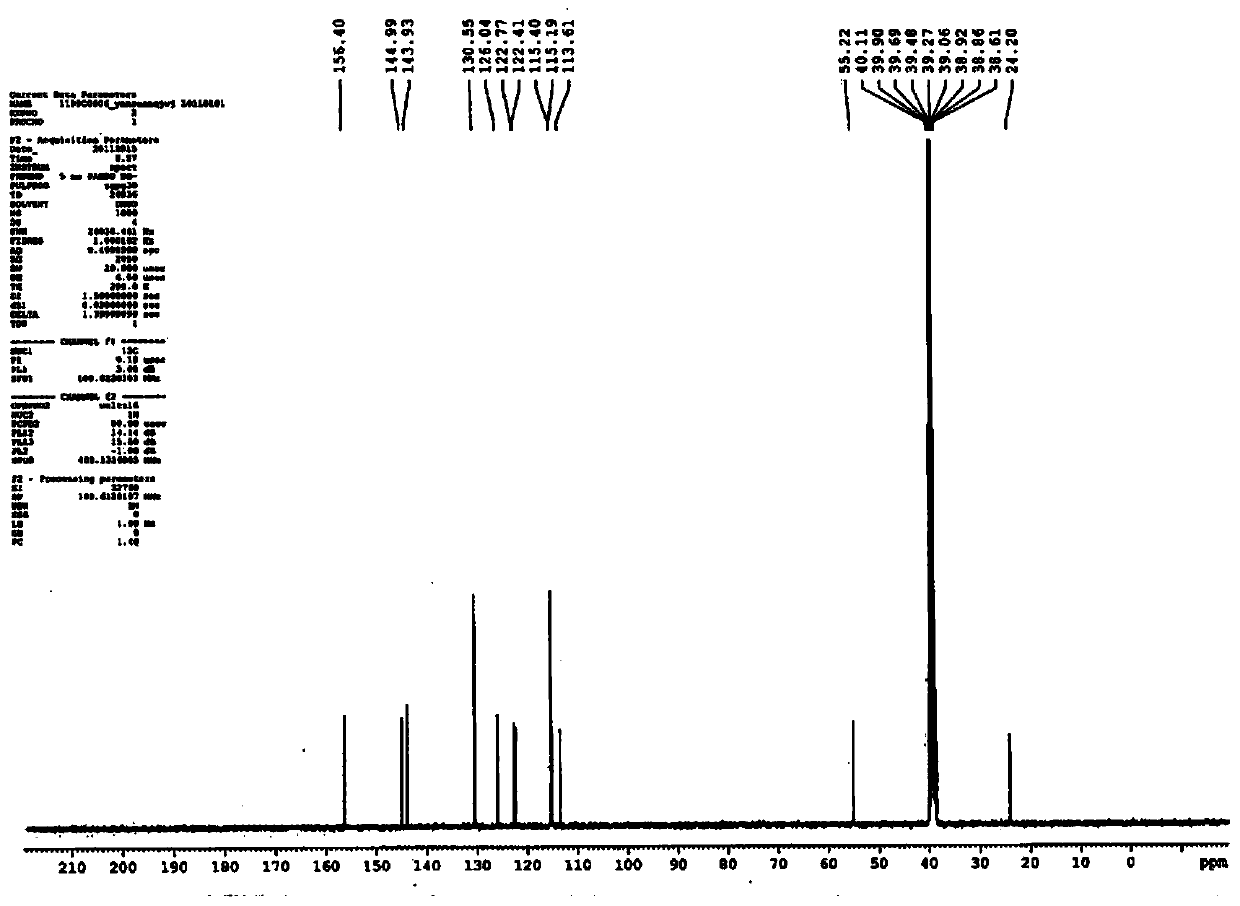
[0078] A superconducting pulsed Fourier transform NMR spectrometer (BRUKEROPTICS, model JY / T007-1996), with deuterated dimethyl sulfoxide (DMSO-D6) as...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com