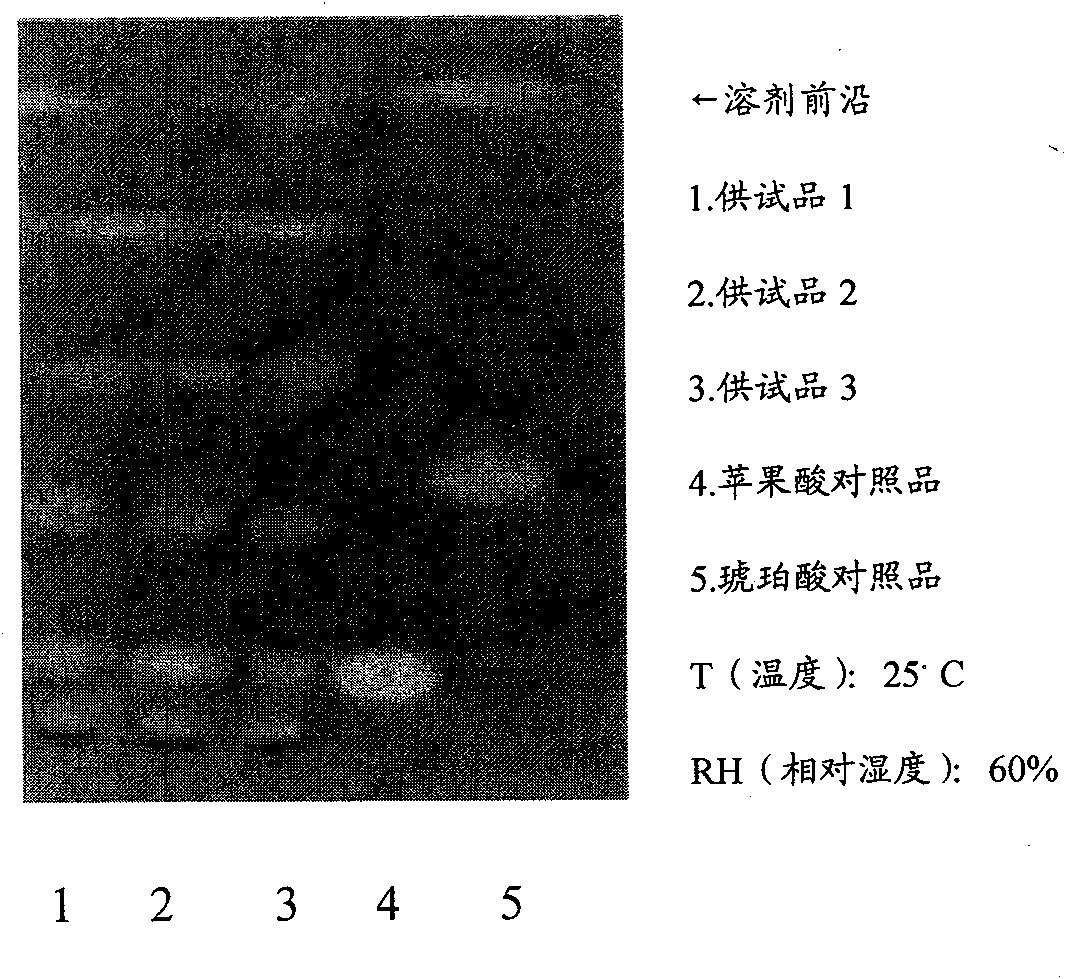
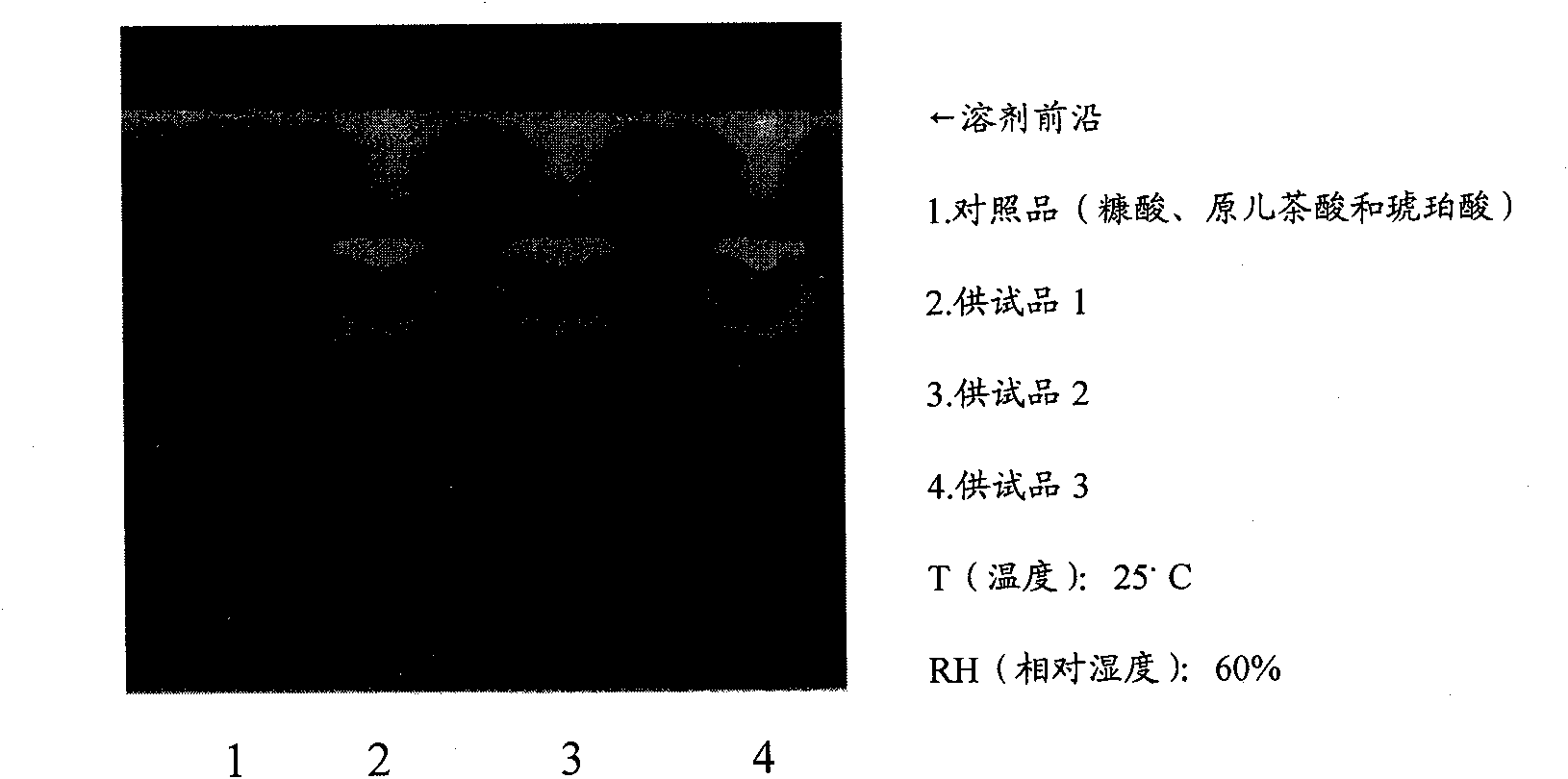
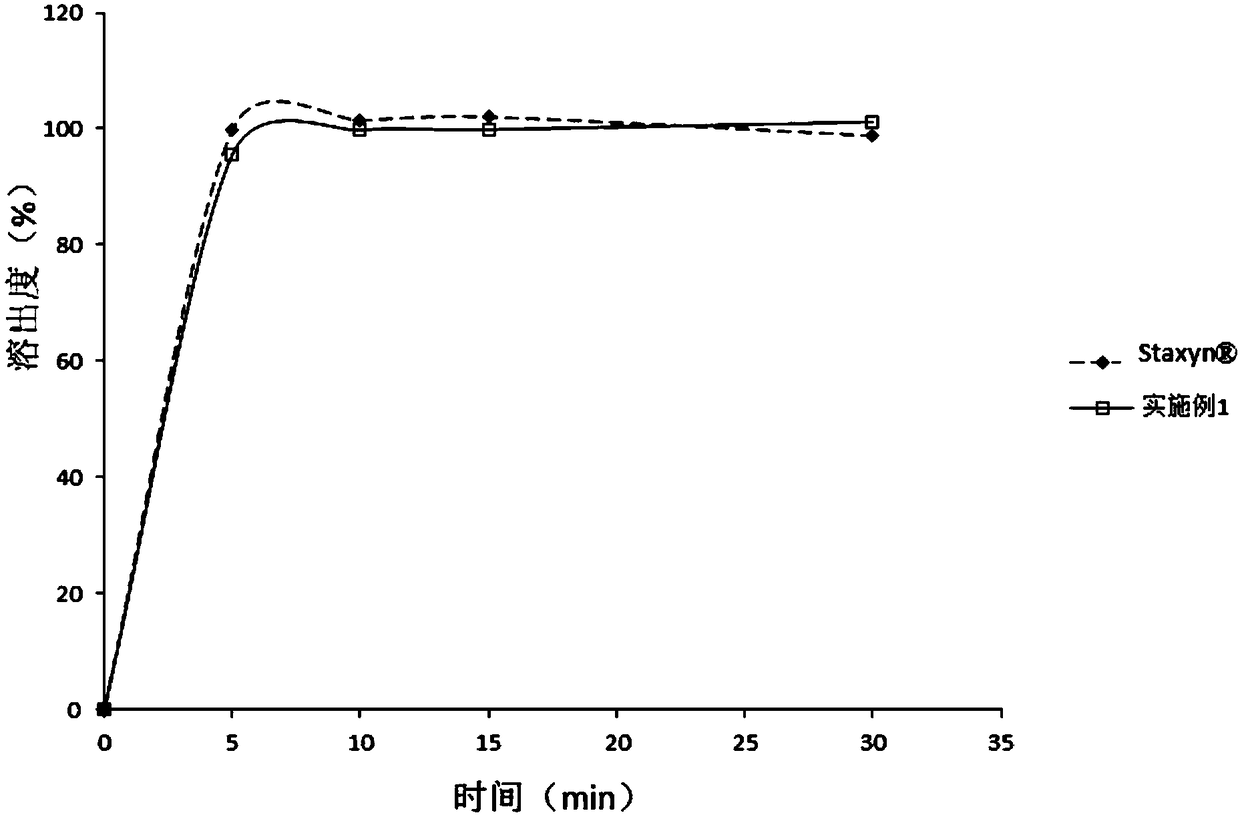
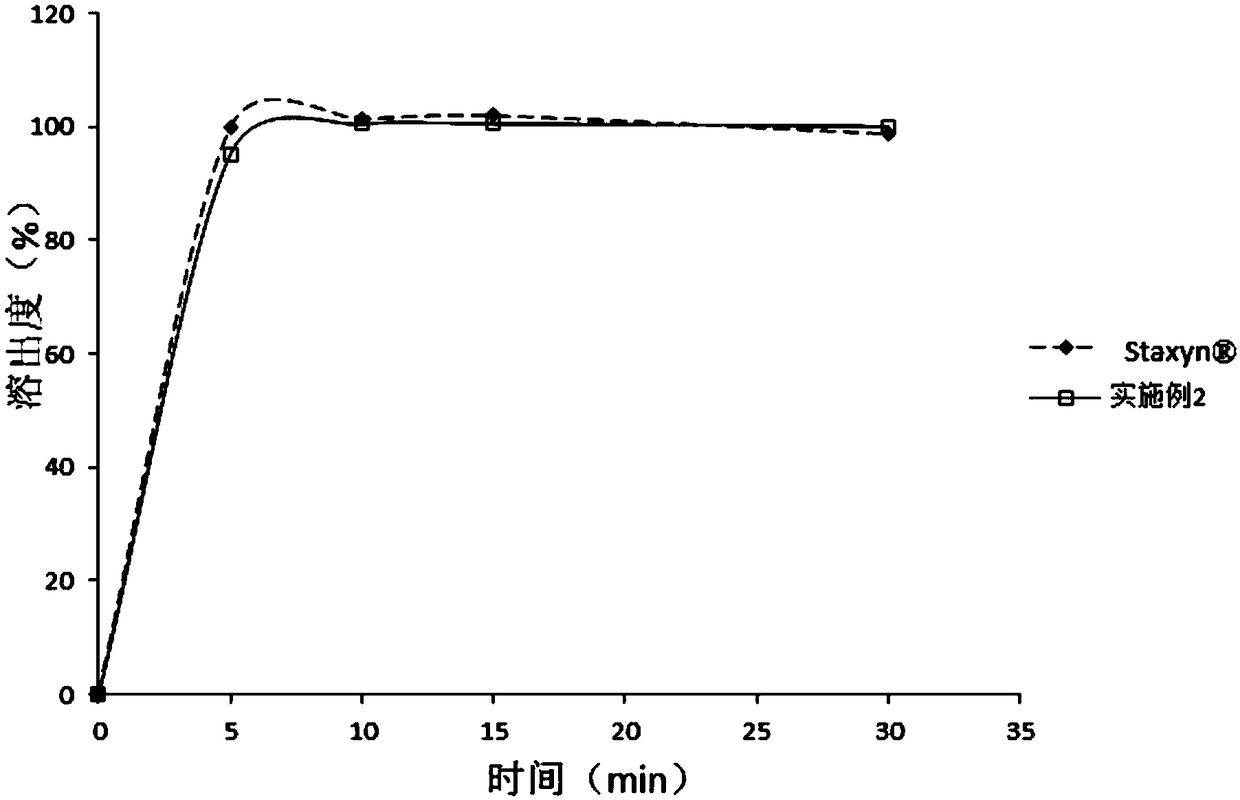
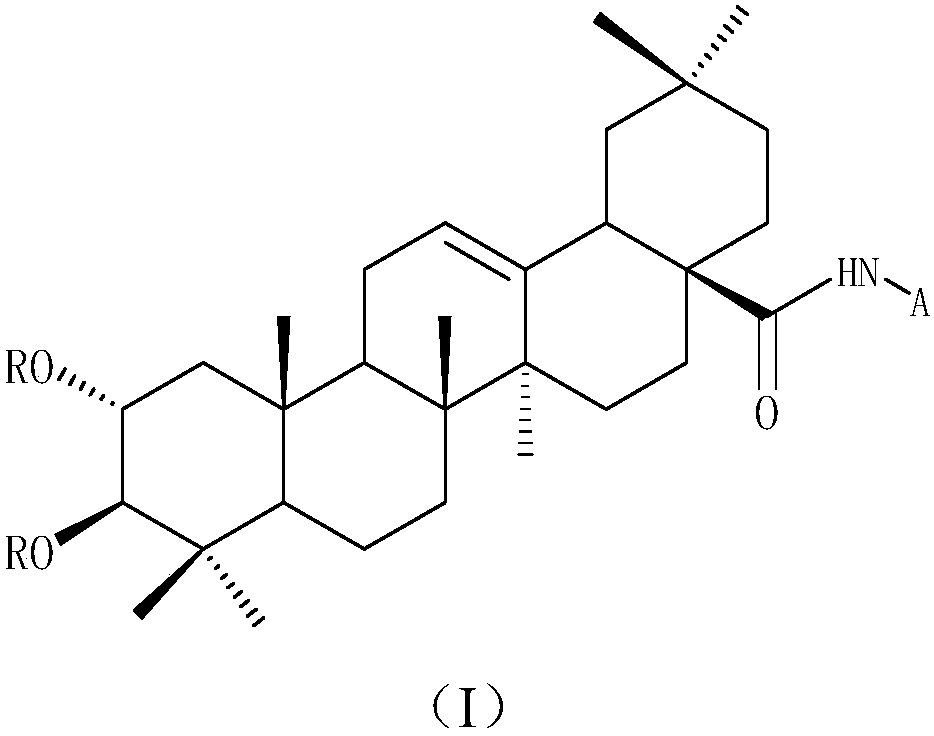
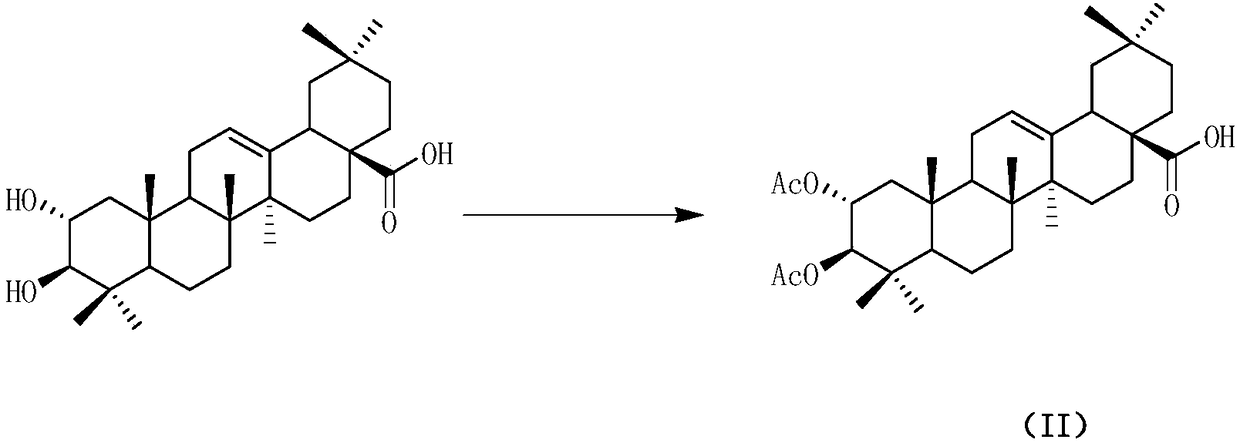
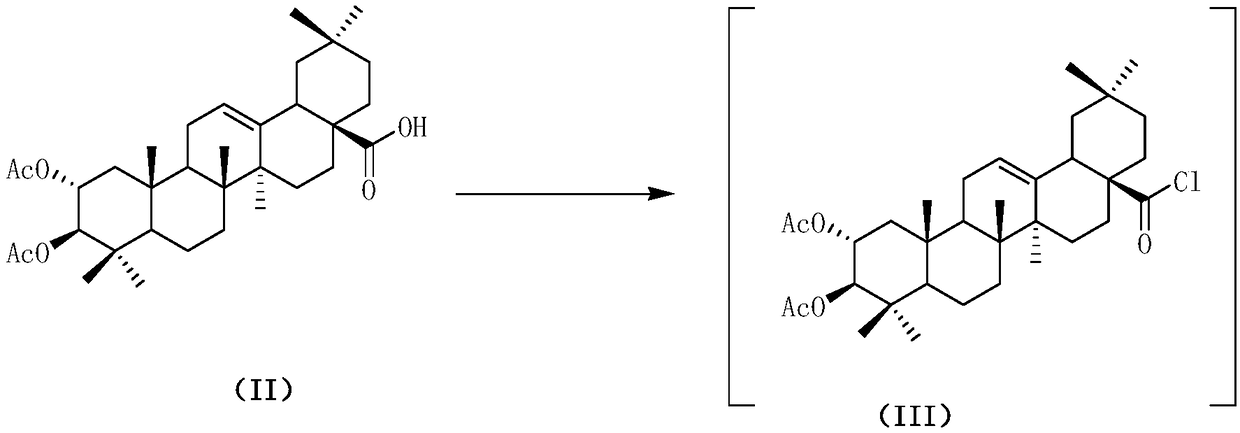
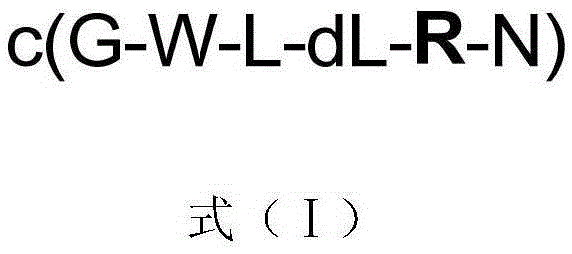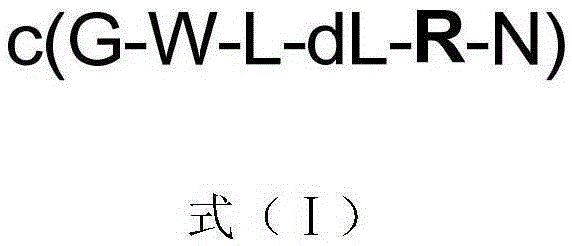Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
60 results about "Reference drug" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Drug Reference Standards. A drug reference standard is a standardized substance which is used as a measurement base for similar substances. Where the exact active substances of a new drug are not known, a reference standard provides a calibrated level of biological effects against which new preparations of the drug can be compared.
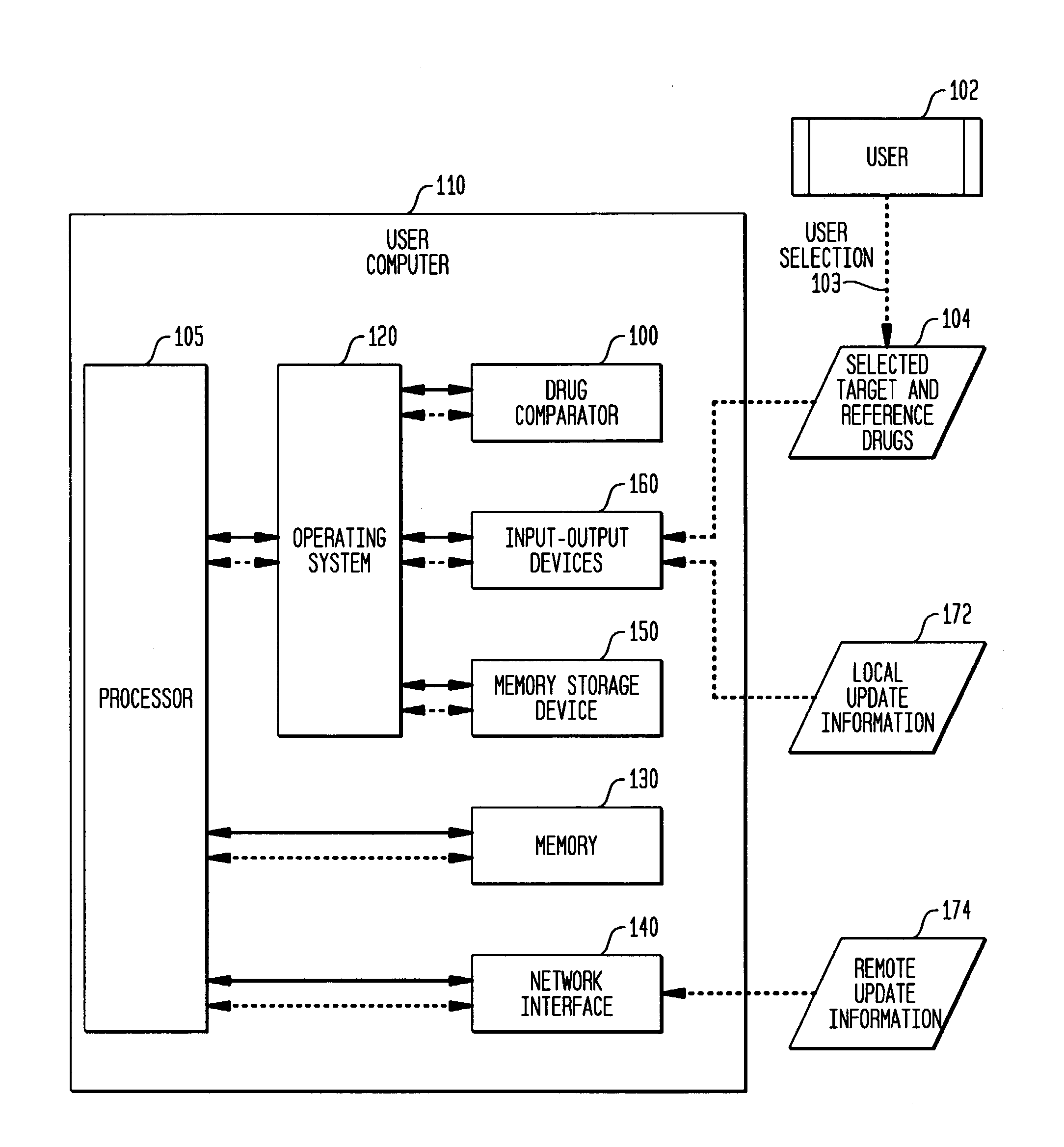
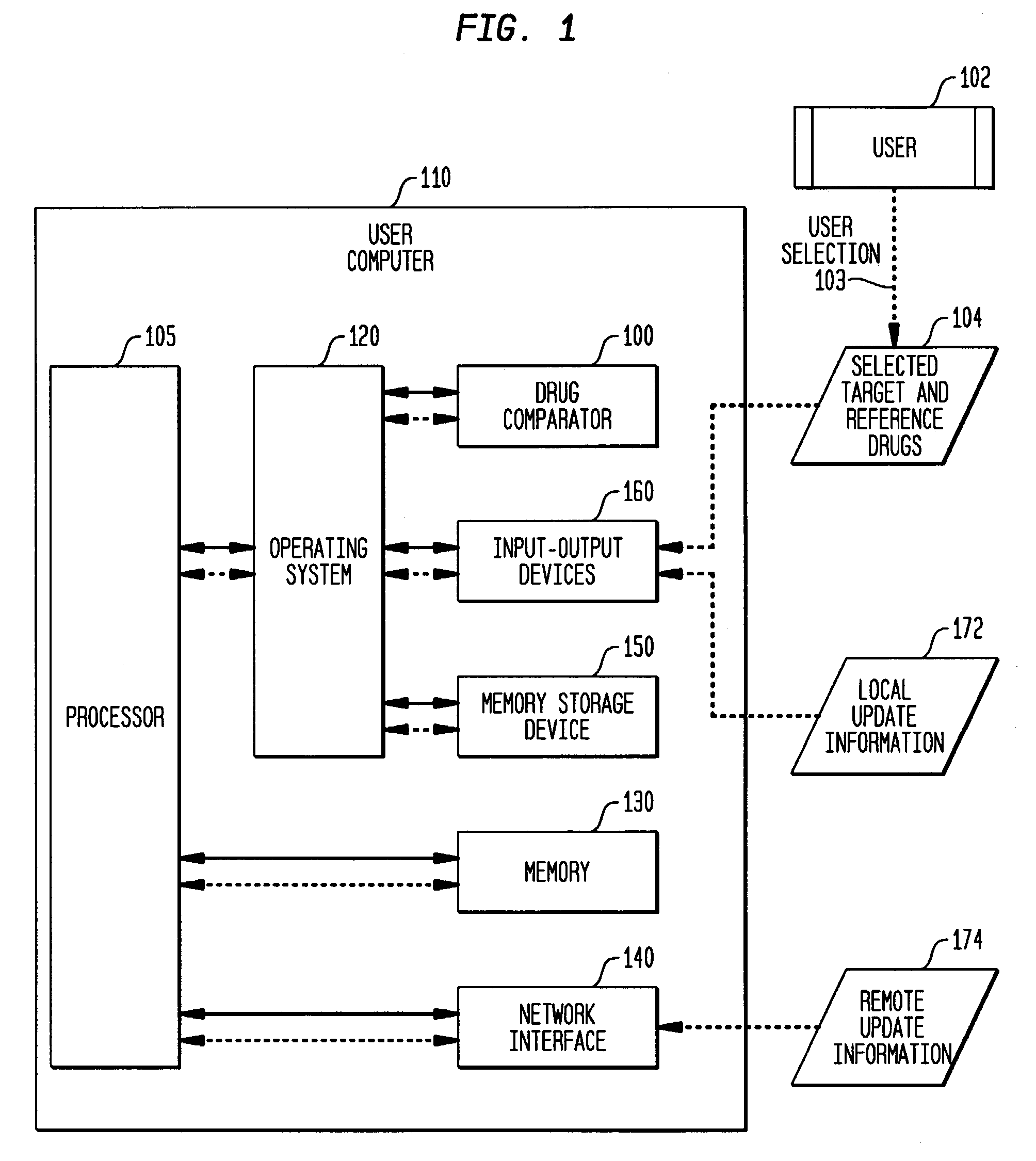
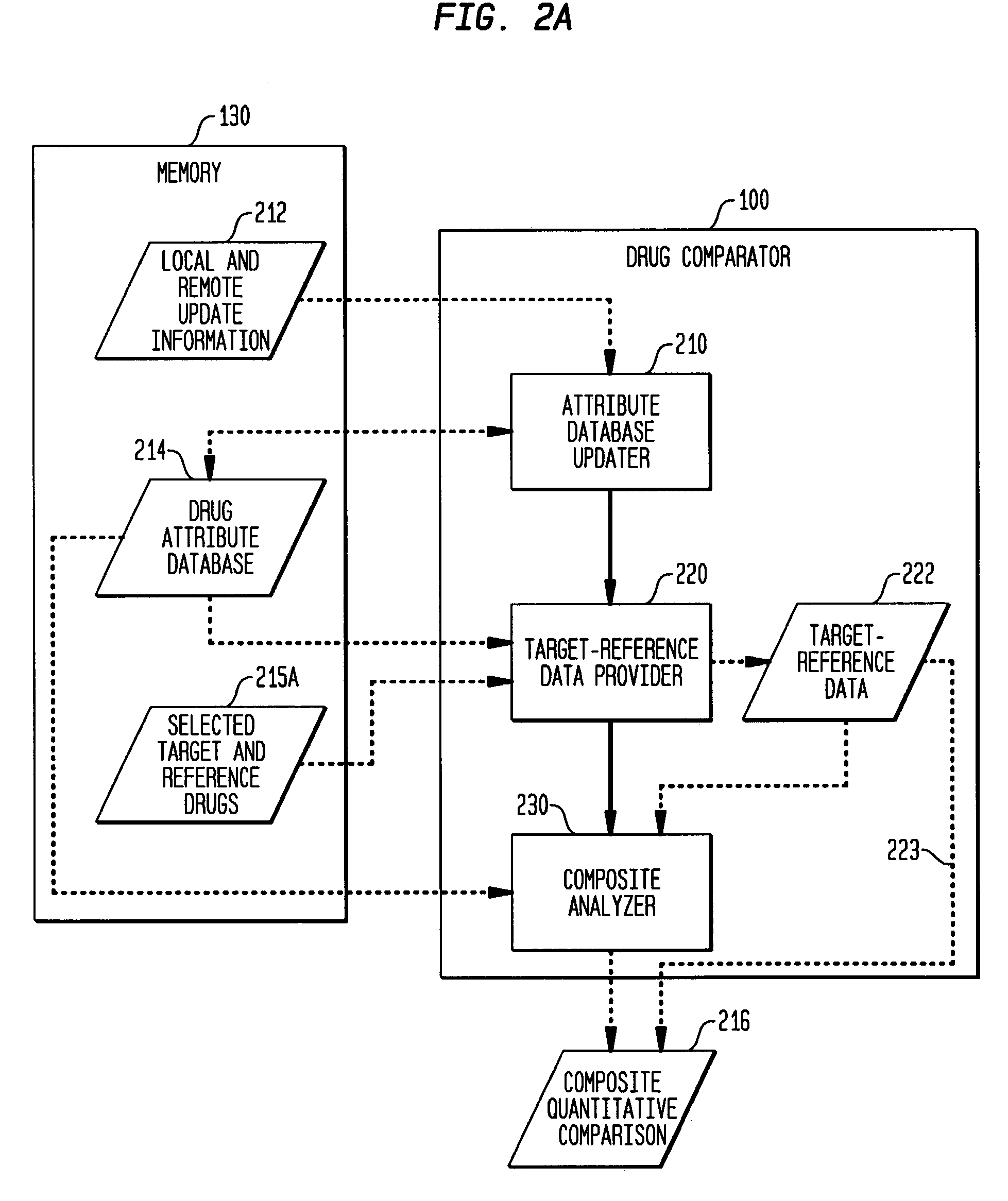
Multi-attribute drug comparison
A computer-implemented apparatus or method, or a software product, for generating a composite quantitative comparison of drug products based on multiple attributes of them. A set of name-attribute similarity scores are generated based on similarities among the names of selected target and reference drugs. A set of product-attribute similarity scores are generated based on similarities among product attributes of the selected target and reference drugs. A target drug confusability score is generated based on the confusability of the target drug as compared to a population of other drugs. The composite quantitative comparison is generated based on a composite of the name-attribute and product-attribute similarity scores, and the target confusability score. A set of one or more severity of confusion scores may also be included in the composite quantitative comparison. These scores are based on one or more indicators of the severity of the consequences to a patient of confusing the target and reference drugs so that, for example, the wrong drug is administered to the patient, or the correct drug is incorrectly administered. The name-attribute similarity scores may be generated based on orthographic, phonetic, and / or phonological analysis. The product-attribute similarity scores may be generated based on the drugs'strengths, indications, dosages, administration routes, manufacturers, pharmacological categories, storage requirements, colors, shapes, legal standing, trademark description, and / or other attributes. The composite quantitative comparison may include severity-weighted similarity scores or both similarity scores and severity of confusion scores. The severity of confusion indicators may include a therapeutic index and / or a contraindication index.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ILLINOIS
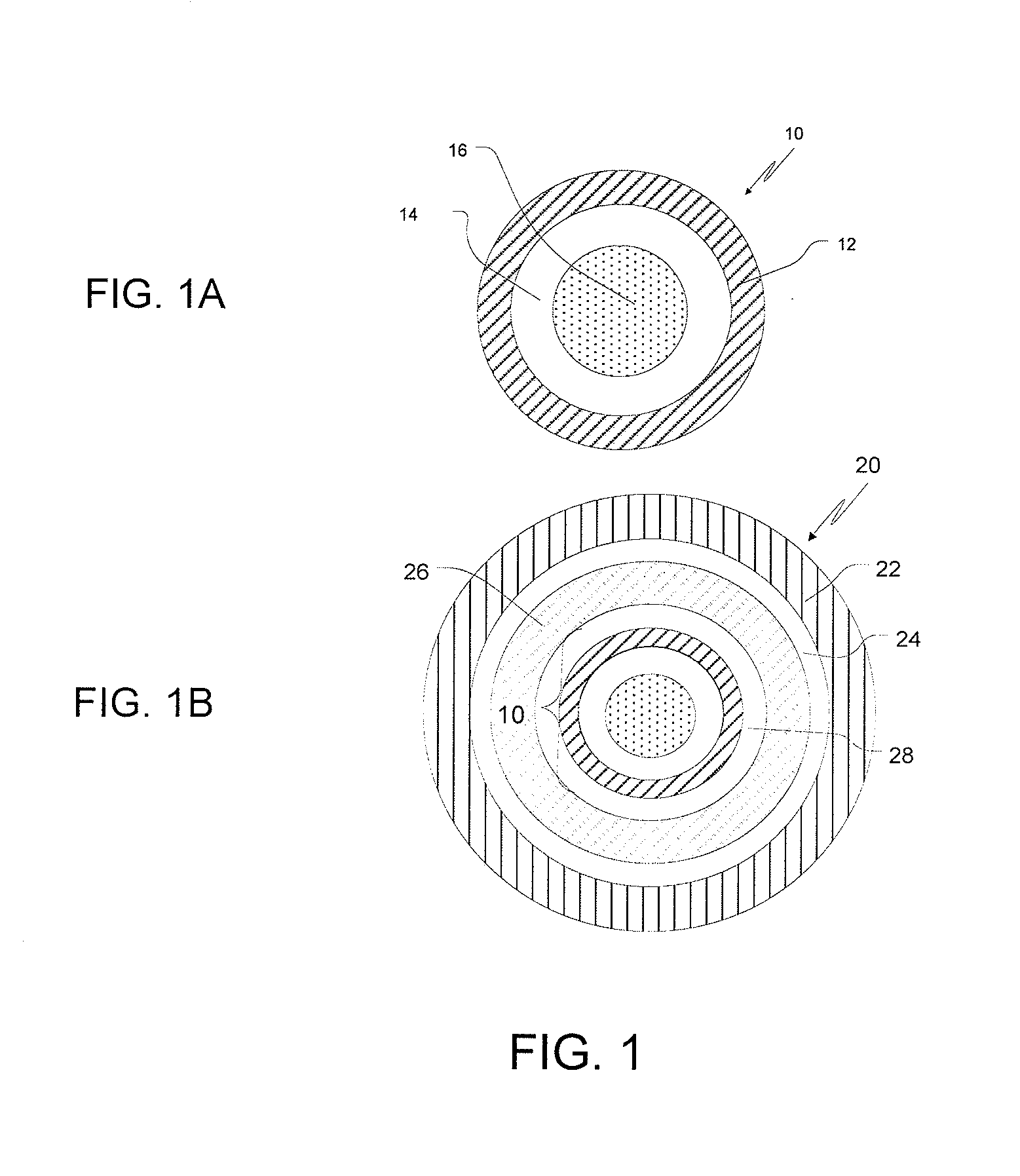
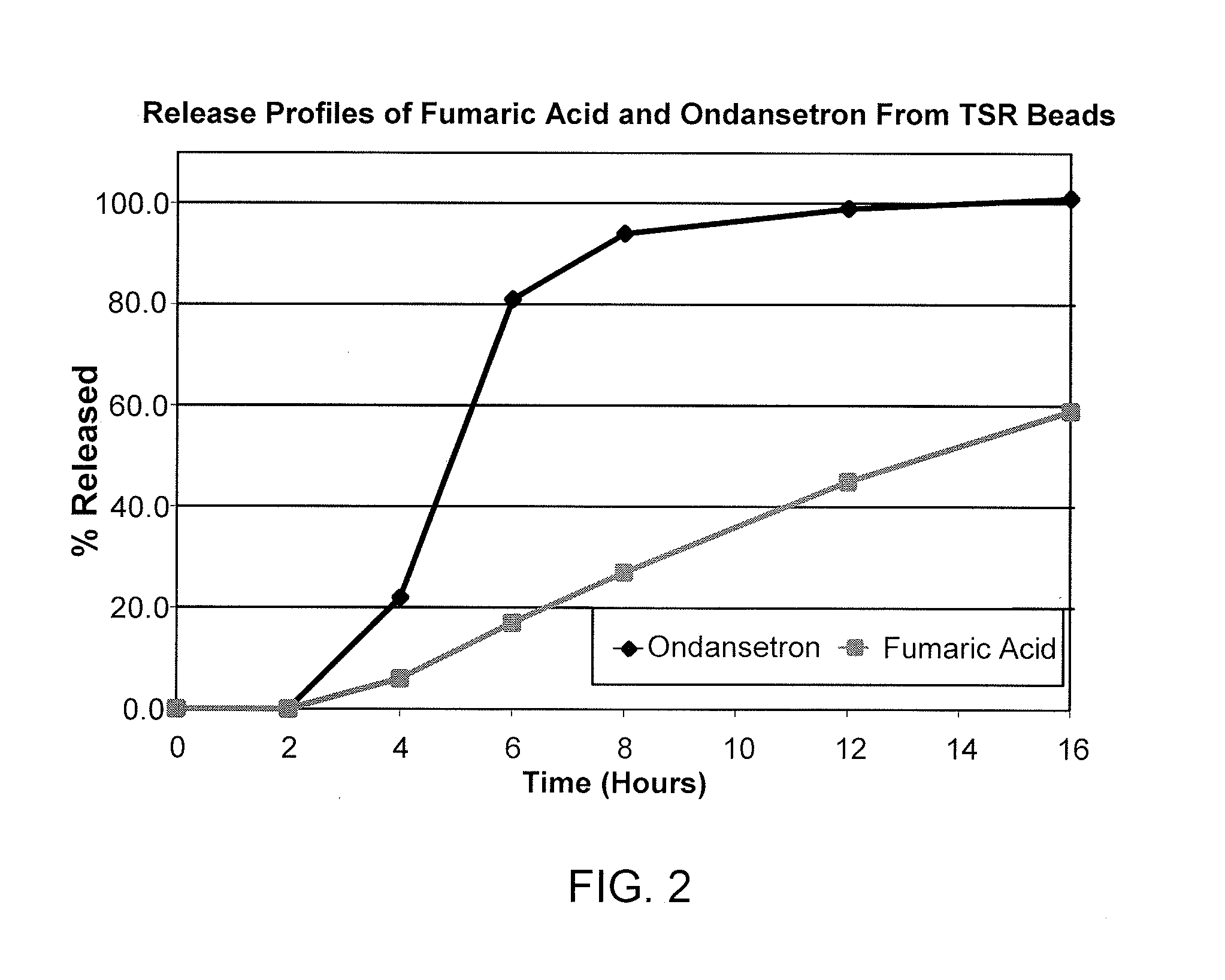
Ondansetron Orally Disintegrating Tablet Compositions for Prevention of Nausea and Vomiting
InactiveUS20110135724A1Disperse fastEasy to swallowOrganic active ingredientsBiocideSerotoninRegimen
This invention is related to a pharmaceutical composition in the patient-friendly orally disintegrating tablet form comprising a weakly basic, selective serotonin 5-HT3 blocking agent for the prevention of nausea and / or vomiting for up to 24 hrs postdosing in cancer patients prior to undergoing moderately emetogenic chemotherapy or partial or whole body radiotherapy or in subjects at moderate to high risk of postoperative or postdischarge nausea and / or vomiting prior to inpatient or outpatient ambulatory surgery. The unit dosage form comprising a multitude of immediate-release drug particles providing dissolution profiles similar to that of reference drug product, and one or more timed, pulsatile-release bead populations, comprising at least one organic acid, which solubilizes said weakly basic selective serotonin 5-HT3 blocking agent prior to releasing it into the hostile intestinal environment, wherein the blocking agent is practically insoluble, is capable of delivering said antiemetic agent in patients in need thereof in a sustained-released fashion to be suitable for a once-daily dosing regimen.
Owner:APTALIS PHARMATECH
Methods for evaluation prognosis and follow-up of drug treatment of psychiatric diseases or disorders
The present invention provides methods for evaluating the pharmacological efficacy of drugs or drug candidates in treatment of psychiatric diseases or disorders, particularly schizophrenia, and for predicting the efficacy of drugs or drug combinations indicated for treatment of both positive and negative symptoms of psychiatric diseases or disorders in an individual having such a disease or disorder. In both methods, the drugs or drug candidates evaluated are assessed for their ability to produce certain changes in the expression of specific genes in peripheral mononuclear cells in blood of psychiatric patients, which are similar to the changes obtained following treatments with reference drugs or drug combinations effective against both positive and negative symptoms of psychiatric diseases or disorders.
Owner:TECHNION RES & DEV FOUND LTD
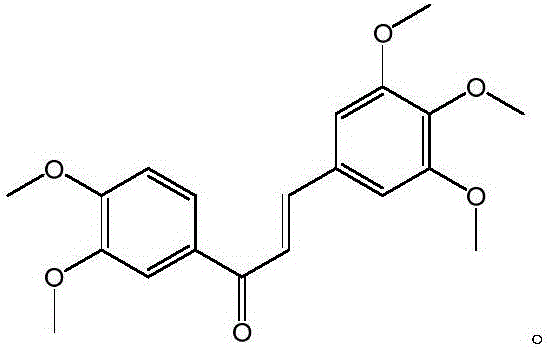
Compound with antitumor activity and preparation method and application of compound
ActiveCN105967991AReduce dosageAvoid destructionOrganic compound preparationCarbonyl compound preparationChalconeTumor cells
The invention discloses a compound with antitumor activity and a preparation method and application of the compound and belongs to the technical fields of new compound synthesis and medicine application. According to the compound, the preparation and the application thereof disclosed by the invention, aromatic aldehyde and aromatic ketone are catalyzed by sodium hydroxide to synthesize 3,4,5-triethoxy-3',4'-dimethoxy chalcone for the first time, and the test of in vitro tumor cell inhibitory activity on the 3,4,5-triethoxy-3',4'-dimethoxy chalcone is realized; the result shows that the compound has higher inhibitory activity for human lung cancer cell A549, human colon carcinoma cell SW620 and human liver cancer cell HepG2. In addition, the antitumor activity of the 3,4,5-triethoxy-3',4'-dimethoxy chalcone for the human colon carcinoma cell SW620 and the human liver cancer cell HepG2 is superior to that of a reference drug 5-fluorouracil. The invention provides a new effective treatment means for tumor treatment and has a broad application prospect.
Owner:HARBIN MEDICAL UNIVERSITY
Medicine for treating inflammation of female reproductive system and preparation and quality control method thereof
InactiveCN101822743AAvoid time costLow cost of reagentsComponent separationAntipyreticSugar Coated TabletDisease
The invention discloses a traditional Chinese medicine for treating inflammation of a female reproductive system and a preparation method thereof as well as a quality control method thereof. The medicine is prepared by the following raw materials in part by weight: 200 to 300 parts of Picria fel-terrae, 350 to 450 parts of herba elephantopi, 350 to 450 parts of Caulis Spatholobi, 350 to 450 parts of Radix zanthoxyli, 350 to 450 parts of membranaceous beautyleaf root, 350 to 450 parts of persimmon leaf and 350 to 450 parts of Thlaspi. The preparation method of the medicine is characterized in that medicinal extracts are used as medicine after being made into dry powder. The medicine is preferably made into sugar coated tablets. At the same time, a quality control method for content measurement and discrimination of the medicine is provided. The general effective rate of the medicine is verified by the clinical test result to be 97.96 percent, and the general effective rate of reference drug is 96.05 percent. After the treatment with the medicine, the disease and the primary symptom are remarkably improved. The medicine has good effect on treating the vaginitis and has slight adverse reaction.
Owner:秦皇岛润青制药有限公司
Detection method for effective components of astragalus Salvia Miltiorrhiza qi-benefiting dropping pill
The invention provides a detection method for effective components of an astragalus Salvia Miltiorrhiza qi-benefiting dropping pill. The method includes identification of panax notoginseng, astragalus and Dalbergia odorifera and tanshinol content determination. Specifically, the method consists of: preparing a panax notoginseng and astragalus test solution, preparing a panax notoginseng and astragalus reference solution, conducting panax notoginseng and astragalus thin-layer identification, preparing a Dalbergia odorifera test solution, preparing a Dalbergia odorifera reference drug solution, performing Dalbergia odorifera thin-layer identification, and determining the tanshinol content by ultra-high performance liquid chromatography. Tests show that the method has very good linearity, repeatability, reproducibility and stability, as well as high recovery rate, and is conducive to more comprehensive control of the quality of the astragalus Salvia Miltiorrhiza qi-benefiting dropping pill.
Owner:TIANJIN TASLY PHARMA CO LTD
TLC identification method for Naoxinqing tablet and persimmon leaf extract
ActiveCN101658546AEasy to prepareEnhanced authenticationComponent separationCardiovascular disorderTest articleFiltration
The invention discloses a TLC identification method for Naoxinqing tablet and persimmon leaf extract. The method includes the following steps: a) a prepared test article is ground and porphyrized, methanol is added, and the mixture obtained is processed by ultrasonication and then is filtered; filter liquor is dried by distillation, water is added to residuals, the mixture obtained is thoroughly stirred so as to dissolve the residuals, filtration is carried out, hydrochloric acid is added to the filter liquor, absolute ether is used for shaking extraction, and an aether layer is separated to be obtained and then is dried by volatilization; the methanol is added for dissolving the residuals, and first test article solution can be obtained; b) proportioned with the chemical ingredients, a persimmon leaf is processed by the operations identical with the step a), and obtained liquor is used as a first reference drug solution; c) a protocatechuic acid reference substance is prepared and then is added with and dissolved in the methanol, and obtained liquor is used as a first reference substance solution; and d) the first test article solution, the first reference drug solution and the first reference substance solution are processed by the TLC analysis. The test article is not the Naoxinqing tablet or the persimmon leaf extract, providing that in the chromatogram of the test article,spots with the same color can not be displayed on corresponding positions on the chromatograms of the reference drug and the reference substance.
Owner:GUANGZHOU BAIYUSN HUTCHISON WHAMPOA CHINESE MEDICINE
Drug composition containing vardenafil hydrochloride and orally disintegrating tablet containing drug composition as well as preparation of orally disintegrating tablet and application of drug composition
ActiveCN108272765AReduce energy consumptionAvoid stratificationPharmaceutical non-active ingredientsPill deliveryDrug contentFiller Excipient
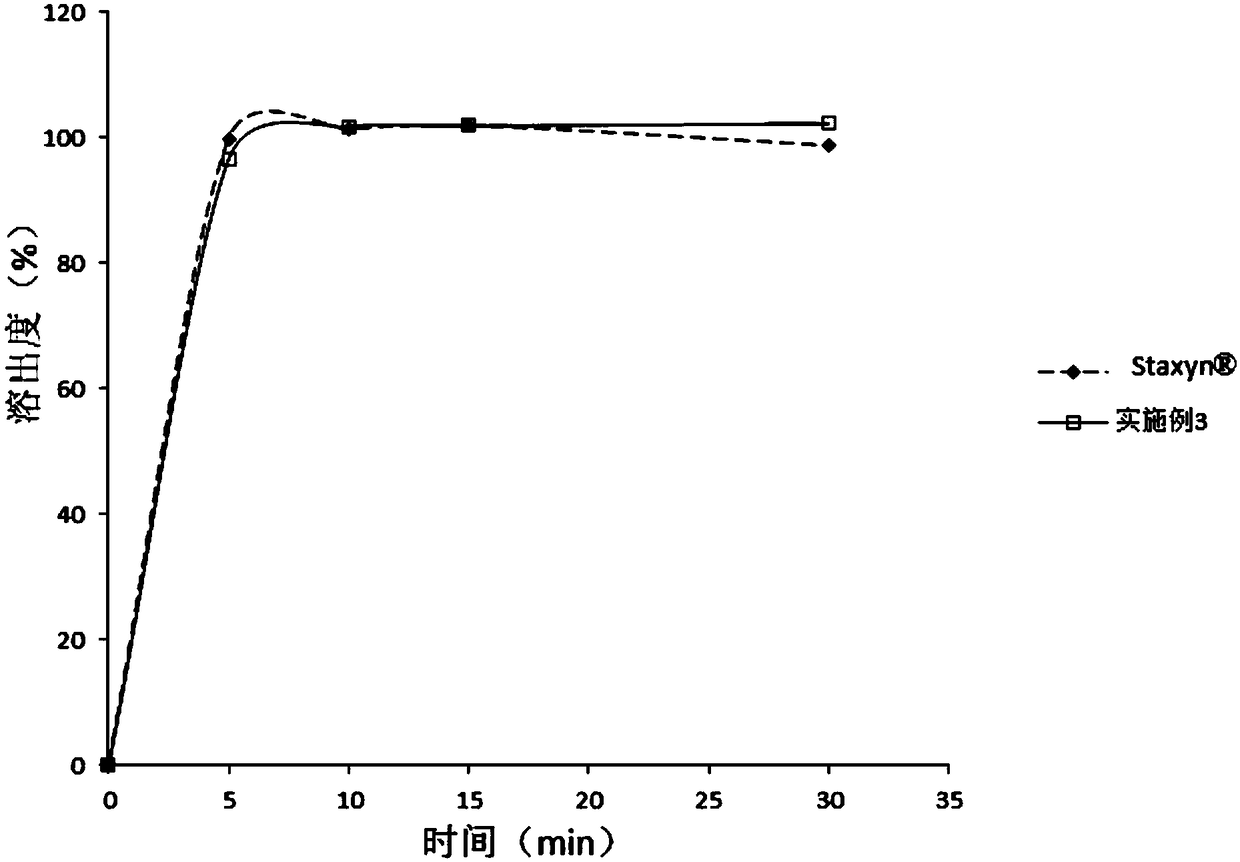
The invention discloses a drug composition containing vardenafil hydrochloride and an orally disintegrating tablet containing the drug composition as well as preparation of the orally disintegrating tablet and application of the drug composition. The drug composition is prepared from an active component, a filling agent and a disintegrating agent, wherein the active component is vardenafil hydrochloride or vardenafil hydrochloride trihydrate; D90 particle diameter of the active component is 45-180 microns. The drug composition is prepared by the conventional auxiliary materials in the field; the large-scale production of the drug composition can be achieved by the existing equipment and auxiliary materials without special auxiliary materials and equipment; the orally disintegrating tabletcontaining the drug composition is higher in drug content uniformity, moderate in hardness and good in administration taste, has stability consistent with that of the reference drug, and can be rapidly disintegrated in the oral cavity.
Owner:江西杏林白马药业股份有限公司
A method for detection of active ingredients in Qishen Yiqi dripping pills
The invention provides a detection method for effective components of an astragalus Salvia Miltiorrhiza qi-benefiting dropping pill. The method includes identification of panax notoginseng, astragalus and Dalbergia odorifera and tanshinol content determination. Specifically, the method consists of: preparing a panax notoginseng and astragalus test solution, preparing a panax notoginseng and astragalus reference solution, conducting panax notoginseng and astragalus thin-layer identification, preparing a Dalbergia odorifera test solution, preparing a Dalbergia odorifera reference drug solution, performing Dalbergia odorifera thin-layer identification, and determining the tanshinol content by ultra-high performance liquid chromatography. Tests show that the method has very good linearity, repeatability, reproducibility and stability, as well as high recovery rate, and is conducive to more comprehensive control of the quality of the astragalus Salvia Miltiorrhiza qi-benefiting dropping pill.
Owner:TIANJIN TASLY PHARMA CO LTD
Propylene tethered ciprofloxacin-isatin hybrids as well as synthetic method and application thereof
InactiveCN108727332AStrong drug resistanceStrong resistanceAntibacterial agentsOrganic active ingredientsMulti-drug-resistant tuberculosisIsoniazid
The invention discloses propylene tethered ciprofloxacin-isatin hybrids as well as a synthetic method and an application thereof. The prepared ciprofloxacin-isatin hybrid 3d have strong resistance toall tested Gram-positive bacteria and Gram-negative bacteria (including pathogens showing strong drug resistance clinically), and efficacy of 3d is equivalent to or even better than the parent ciprofloxacin or levofloxacin; antibacterial activity of hybrid 3b (MIC: 0.10 and 0.5 mu g / mL) for an mycobacterium tuberculosis H37Rv strain is 4 times and 8 times that of ciprofloxacin (MIC: 0.78 mu g / mL)and rifampicin (MIC: 0.39 mu g / mL), and antibacterial activity of the hybrid 3b for multi-drug resistant tuberculosis is 4->256 times that of three reference drugs including ciprofloxacin (MIC: 2.0 mug / mL), rifampicin (MIC: 32 mu g / mL) and isoniazid (>128 mu g / mL). The hybrids 3b and 3d with lower cytotoxicity (CC50: 64 and 256 mu g / mL) show acceptable metabolic stability and in-vivo PK (pharmacokinetics) characteristics.
Owner:王若
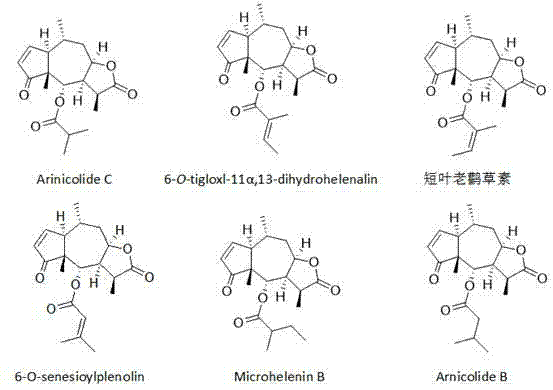
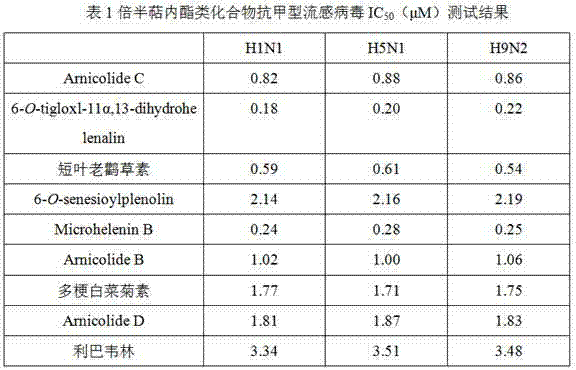
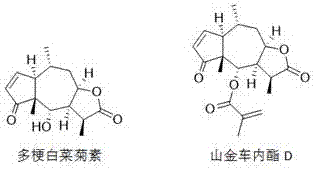
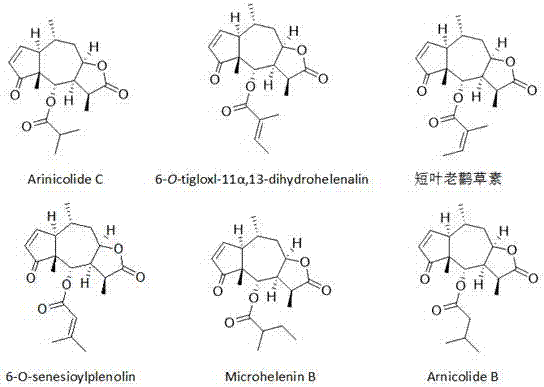
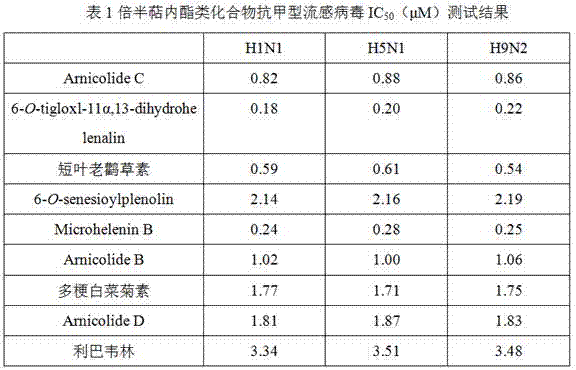
Application of sesquiterpene lactone compounds in preparation of anti-influenza virus drugs
InactiveCN104771393AHas anti-influenza virus effectGood anti-influenza virus activityOrganic active ingredientsOrganic chemistryReference drugDrug
The invention belongs to the field of medicines, and specifically discloses applications of sesquiterpene lactone compounds in the preparation of anti-influenza virus drugs. The anti-influenza virus drugs contain sesquiterpene lactone compounds with an effective dose and pharmaceutically-acceptable carriers. The invention finds that sesquiterpene lactone compounds have an anti-influenza virus effect, and novel compounds are provided for the preparation of anti-influenza virus drugs. The provided sesquiterpene lactone compounds have a very good anti-influenza virus activity, and the experiment results show that the anti-influenza virus IC50 value of the sesquiterpene lactone compounds is smaller than that of a positive reference drug ribavirin.
Owner:JINAN UNIVERSITY
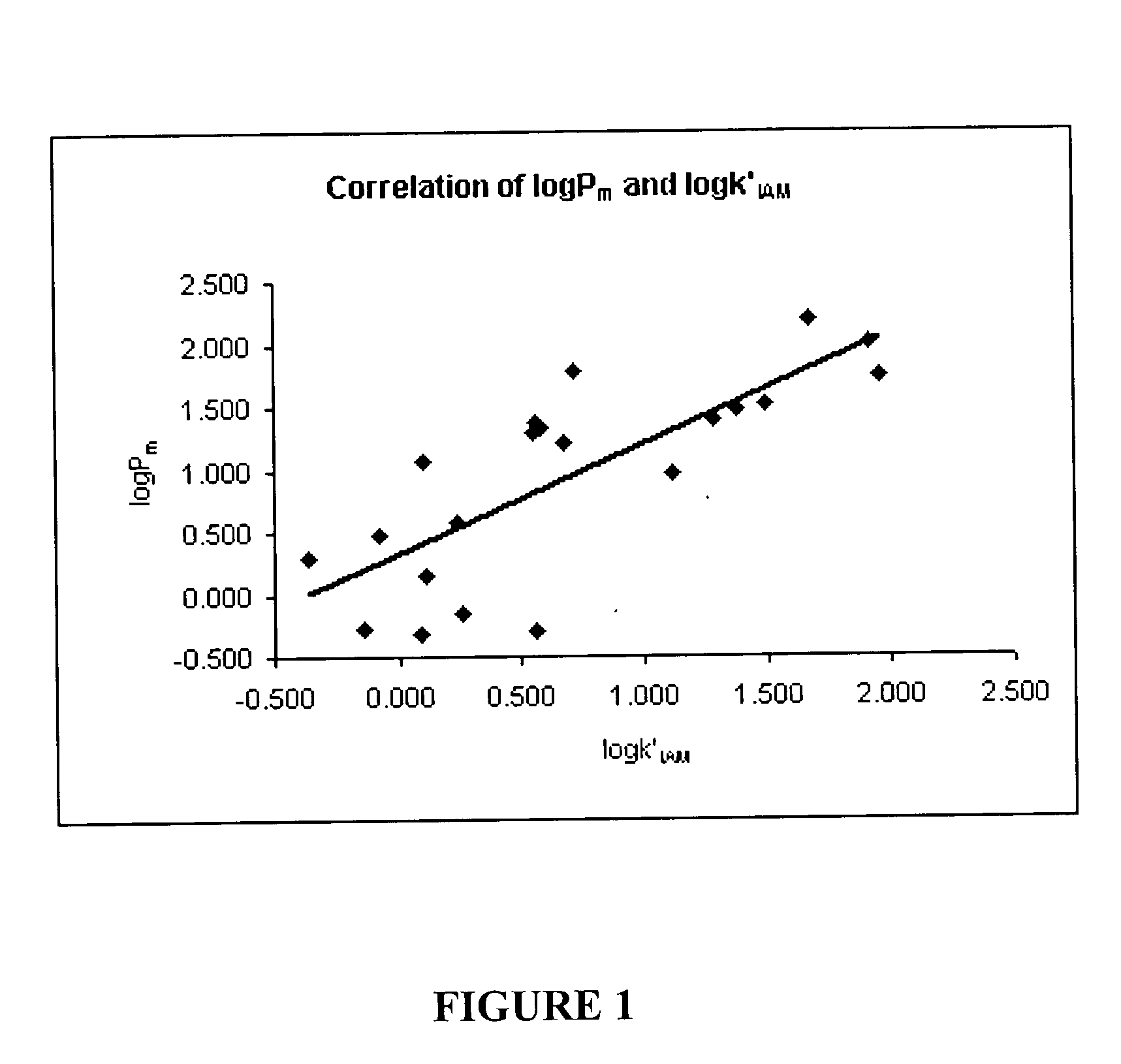
In vitro method for determining drug permeability using immobilized artificial membrane chromatography
InactiveUS20050014280A1Inexpensive and convenientConvenient and inexpensive methodAnalysis using chemical indicatorsComponent separationMedicineRetention time
The present invention provides a method for determining drug permeability, wherein the method comprises, for each reference drug of at least two reference drugs having (i) the same ionization state at a given pH and (ii) a known drug permeability determined by the same drug permeability testing technique, the calculation of a metric related to retention time of each reference compound eluted from a liquid chromatography column having an immobilized artificial membrane functional group. Then, based on each metric and each known drug permeability, drug permeability is expressed as a function of the metric. The method may further comprise the calculation of the metric related to retention time for a further drug eluted from the liquid chromatography column, the further drug having the same ionization state at the given pH and an unknown drug permeability, and an assessment of drug permeability for the further drug, based on the metric for the further drug and the function.
Owner:PATHEON
Method for identifying agarwood
InactiveCN109541116AImprove detection accuracyGood reproducibilityComponent separationAgarwoodSolvent
The invention discloses a method for identifying agarwood. The method comprises the following steps of: S1: preparing a test solution: taking 1g of test agarwood powder, adding 20-30 mL of ethanol solution, performing sonicating for 50-70 min, performing filtering, and diluting the filtrate to a volume of 25mL to prepare the test solution; S2: preparing a reference drug solution and a reference substance solution, wherein the reference drug solution is prepared by taking agilawood medicinal materials with 0.5g to perform the step S1 to prepare the reference drug solution, and the reference substance solution is prepared by taking methanol solution of 6,7-dimethyl-2(2-phenethyl)chromone to prepare the reference substance solution containing 0.05mg in each 1mL; and S3: performing thin layerchromatographic detection to absorb each of three solutions for 5[Mu]L which are respectively dispensed on the same silica gel GF254 thin layer plate, taking the methyl alcohol-glacial acetic acid asa developing solvent to perform development, taking-out and drying, and performing viewing under an ultraviolet lamp and the sunlight. Besides, the method provided by the invention is safe to operate,better in reproducibility and more complete in the chromatography information.
Owner:北京三和药业有限公司
Application of sesquiterpene lactone compounds in the preparation of anti-influenza virus drugs
InactiveCN104771393BHas anti-influenza virus effectGood anti-influenza virus activityOrganic active ingredientsOrganic chemistryIc50 valuesMedicine
The invention belongs to the field of medicines, and specifically discloses applications of sesquiterpene lactone compounds in the preparation of anti-influenza virus drugs. The anti-influenza virus drugs contain sesquiterpene lactone compounds with an effective dose and pharmaceutically-acceptable carriers. The invention finds that sesquiterpene lactone compounds have an anti-influenza virus effect, and novel compounds are provided for the preparation of anti-influenza virus drugs. The provided sesquiterpene lactone compounds have a very good anti-influenza virus activity, and the experiment results show that the anti-influenza virus IC50 value of the sesquiterpene lactone compounds is smaller than that of a positive reference drug ribavirin.
Owner:JINAN UNIVERSITY
Pharmaceutical composition containing vardenafil hydrochloride, orally disintegrating tablet and preparation and application thereof
ActiveCN108272765BGood disintegrationImprove solubilityPharmaceutical non-active ingredientsPill deliveryDrug contentOrally disintegrating tablet
Owner:江西杏林白马药业股份有限公司
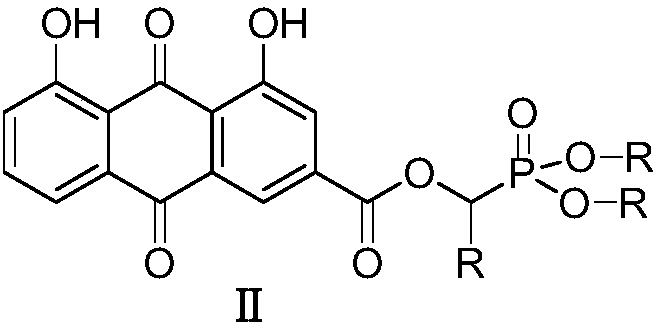
Rhubarb acid ester derivative and preparation method and application thereof
ActiveCN109053798AImprove antibacterial propertiesNovel structureAntibacterial agentsOrganic active ingredientsAntimicrobial actionAntibacterial activity
The invention discloses a rhubarb acid ester derivative in the field of antibiotic compounds. The chemical structural formula of rhubarb acid ester is as shown in the formula (II), the formula (II) isshown in the description, wherein R is a chain substituent group of 1-5 carbon atoms or a naphthenic base or an aromatic hydrocarbon group of 3-6 carbon atoms. An antibiotic activity test proves thatthe rhubarb acid ester derivative with the novel structure has the antibiotic activity on methicillin-resistant staphylococcus aureus (MRSA). A plurality of targets have the outstanding antibiotic effect on methicillin-resistant staphylococcus aureus (MRSA), are superior to the reference drug oxacillin and is close to the reference drug vancomycin, and the rhubarb acid ester derivative can be served as a candidate compound to resist methicillin-resistant staphylococcus aureus (MRSA) and be researched.
Owner:ZUNYI MEDICAL UNIVERSITY
Thin-layer chromatographic detection method of curcuma zedoary in Fushengkang tablet
InactiveCN107328890ASolve gelatinizationSolve stickinessComponent separationThin layer chromatographicColor tests
The invention discloses a thin-layer chromatographic detection method of curcuma in Fushengkang tablets. This method uses ethyl acetate to extract the volatile oil in Fushengkang as the test solution; and uses the same method to process the reference medicinal material Curcuma to make a reference medicinal solution; petroleum ether (60-90°C)-ethyl acetate-glacial acetic acid is used as Developing agent, use silica gel thin-layer plate to develop, spray with newly prepared vanillin test solution, heat at 105°C until the spots are clear in color; If there are no spots, it means that the finished product of Fushengkang contains zedoary ingredients; and if the chromatogram of the test product does not show the main spot of the same color at the position corresponding to the chromatogram of the reference medicinal material, it means that the finished product of Fushengkang does not contain zedoary ingredients. The present invention uses ethyl acetate to reflux heat the sample instead of using ether to sonicate the sample, which solves the problems of sample gelatinization, adhesion, and difficult filtration, and reduces the potential harm to human health; the color development result is accurate: the spots are clear and reproducible Good performance and high accuracy.
Owner:青岛华仁太医药业有限公司
S-triazolo-thiadiazole and thiadiazine derivatives, preparation method and application thereof
InactiveCN105001241AInhibitory Activity EquivalentAntibacterial agentsOrganic active ingredientsShikimate dehydrogenaseThiadiazoles
The invention relates to S-triazolo-thiadiazole and thiadiazine derivatives and an application thereof. Research results show that, the minimal inhibitory concentration (MIC) of the derivatives to mycobacterium tuberculosis standard strains H37Rv is 0.25 [mu]g / mL, and the inhibitory activities of the derivatives to isoniazide / rifampicin-resistant mycobacterium tuberculosis (MDRTB) and rifampicin-resistant mycobacterium tuberculosis (RDRTB) are the same and the MIC of the derivatives to MDRTB and RDRTB is 0.25-4 [mu]g / mL. Meanwhile, the resistance of some derivatives to MDRTB or RDRTB is better than the resistance of the derivatives to reference drugs RIF and INH. At the same time, most derivatives are equivalent in inhibitory activity to Shikimate dehydrogenase, thus being good in development prospect.
Owner:MEDICINE & BIOENG INST OF CHINESE ACAD OF MEDICAL SCI
Cyclic hexadepsipeptide compound and application thereof in preparing benign prostatic hyperplasia resistant drug
InactiveCN105294842AEnhanced inhibitory effectPeptidesCyclic peptide ingredientsStructural formulaHypotype
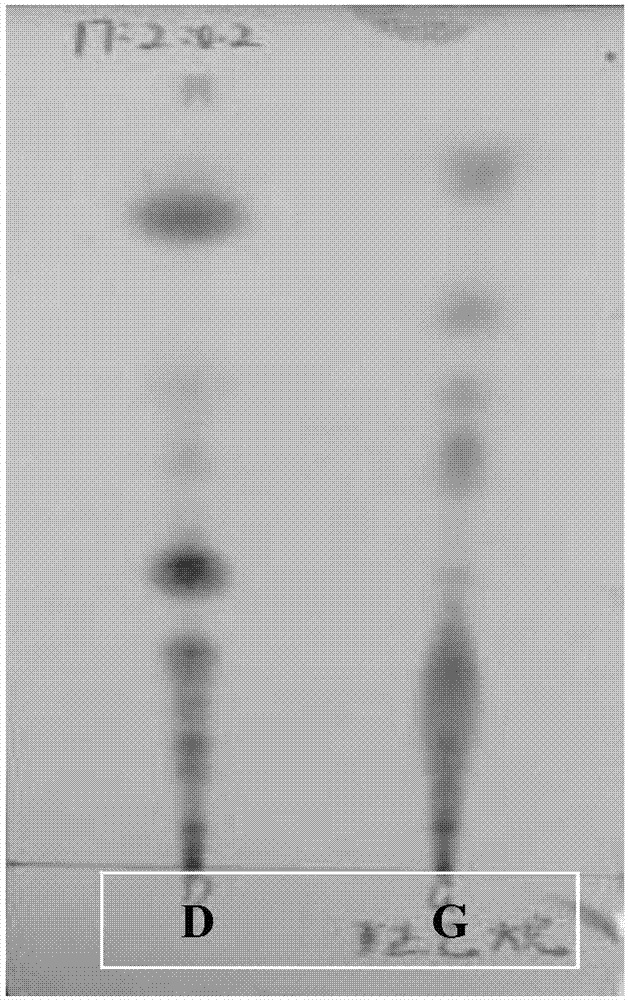
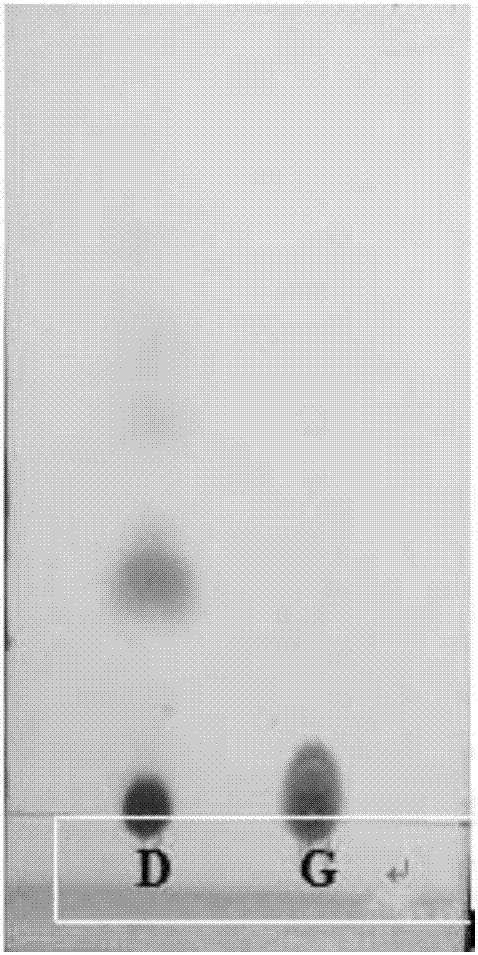
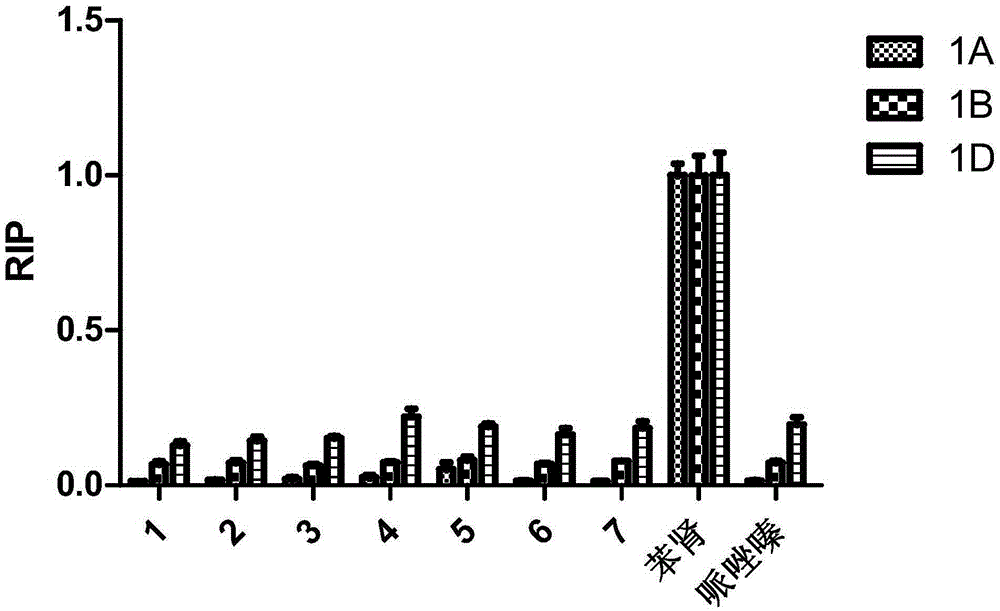
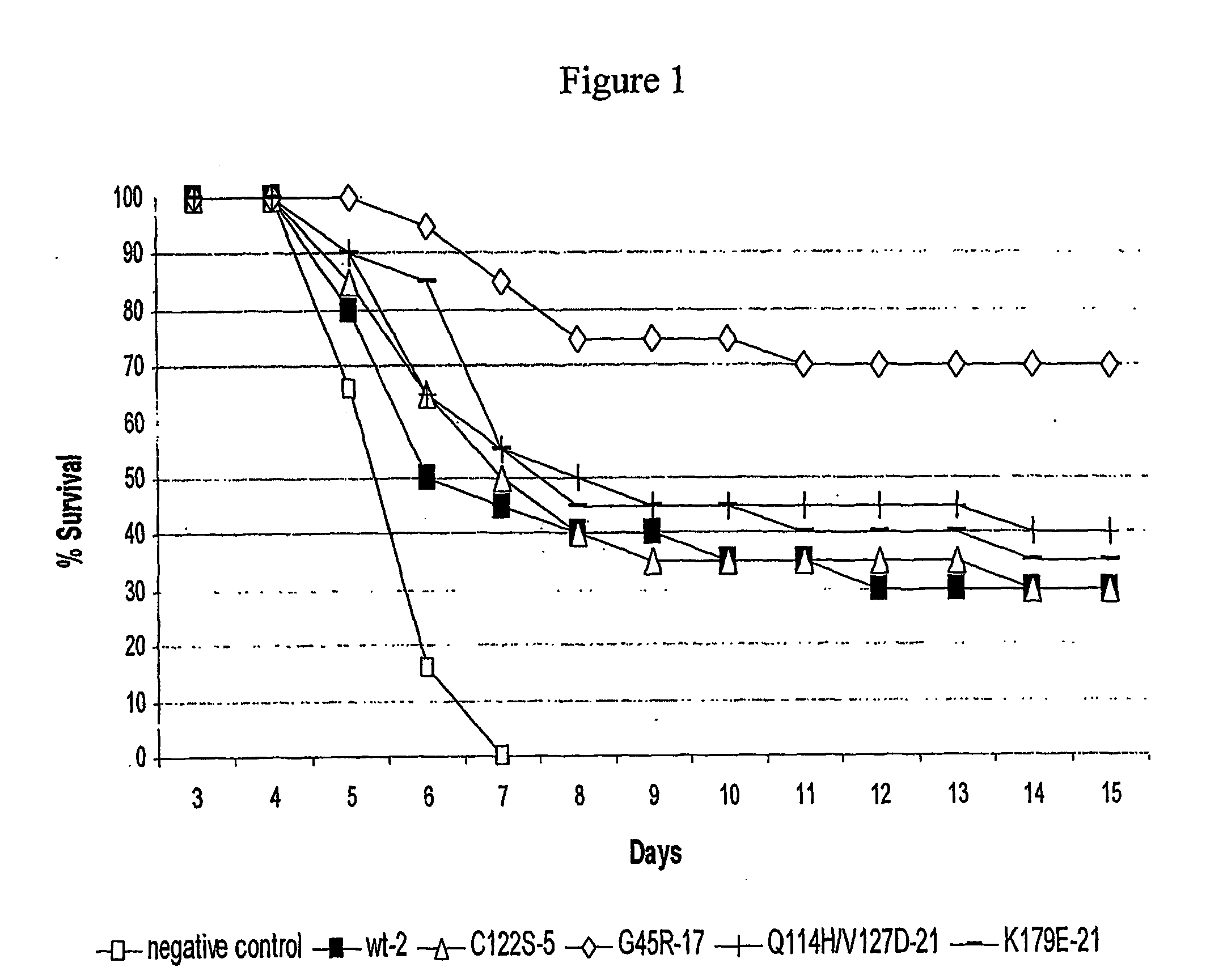
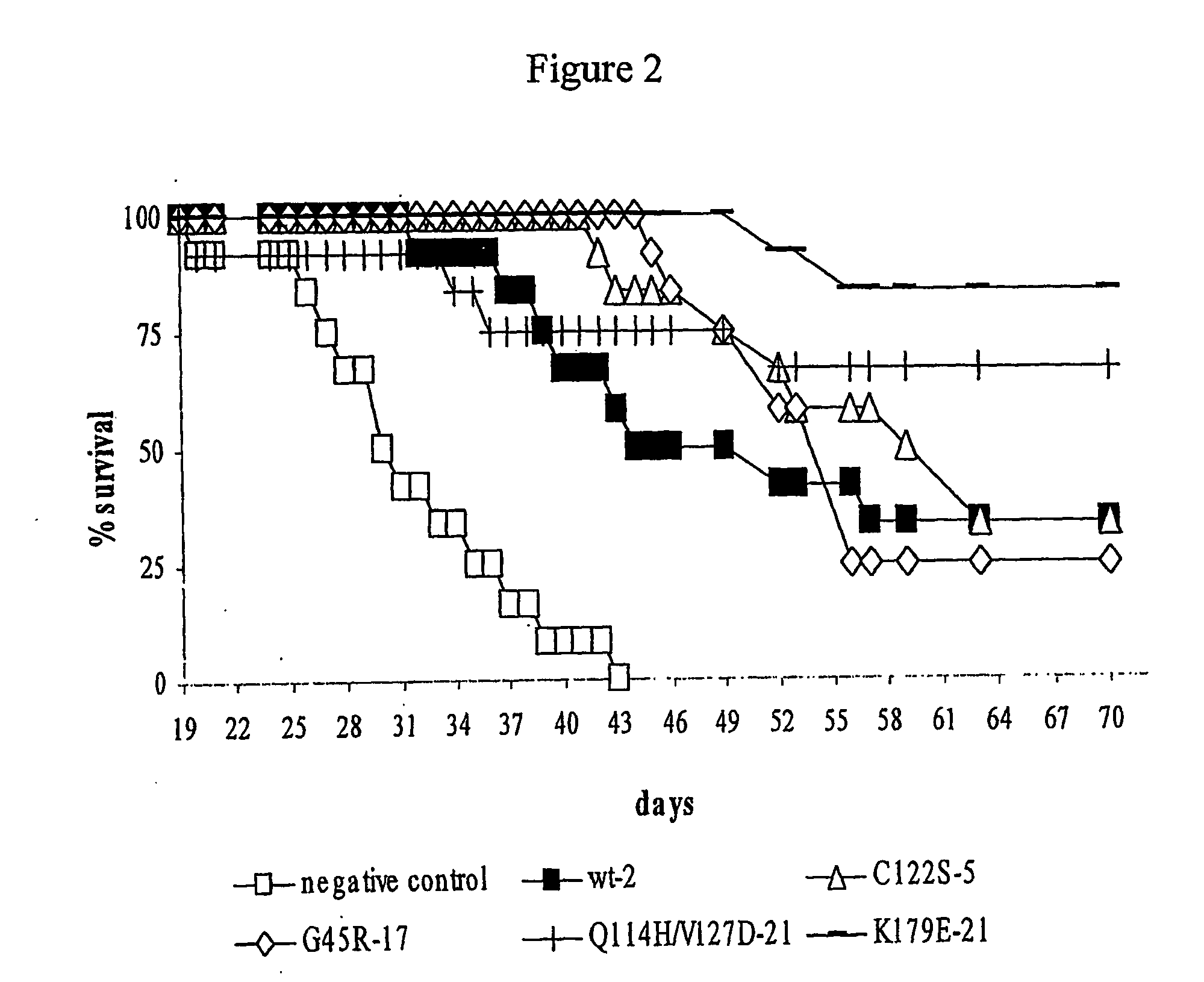
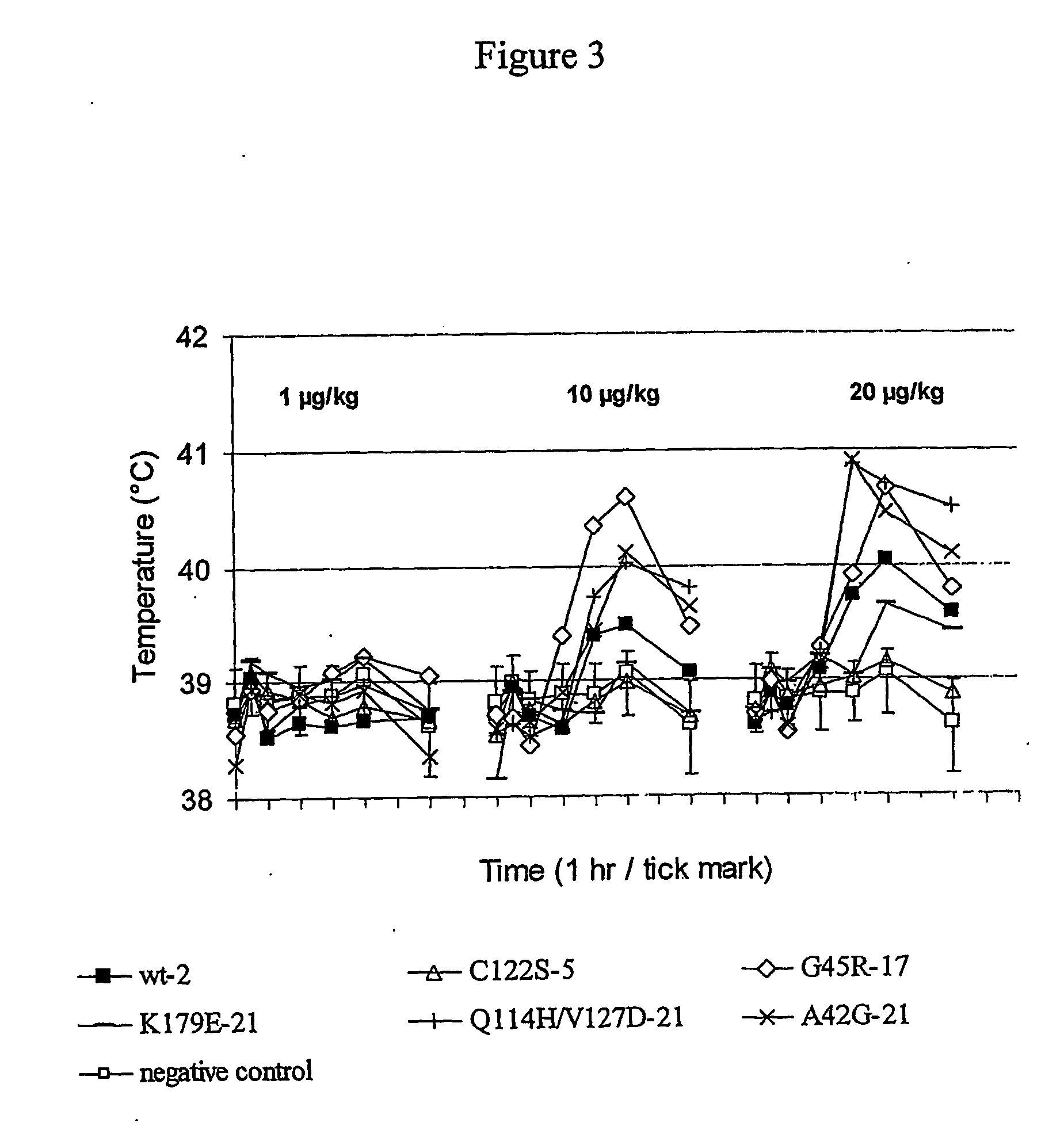
The invention discloses a kind of cyclic hexadepsipeptide compound and an application thereof in preparing a benign prostatic hyperplasia resistant drug. The structural formula of the cyclic hexadepsipeptide compound and a salt thereof for the drug are shown as c(G-W-L-dL-R-N) (formula (I)), wherein in the formula (I), the compound 1: R is equal to Ser / S; compound 2: R is equal to Asp / D; the compound 3: R is equal to Glu / E; the compound 4: R is equal to His / H; or the compound 5: R is equal to Met / M. The cyclic hexadepsipeptide compound has good nonselective anti-junction effect for three hypotypes of alpha1-AR, namely alpha1A-, alpha1B- and alpha1D-AR, for partial hypotypes, the effect is better than a positive reference drug prazosin, and an important significance for developing the benign prostatic hyperplasia resistant drug can be realized..
Owner:SOUTH CHINA SEA INST OF OCEANOLOGY - CHINESE ACAD OF SCI
Method for providing natural therapeutic agents with high therapeutic index
Methods for identifying and providing new therapeutic agent(s) by selecting at least one polypeptide encoded by a natural allelic variant of one preselected gene having a therapeutic potential; determining the therapeutic index of the selected polypeptide(s) and retaining as therapeutic agent(s) those polypeptide(s) whose therapeutic index is higher than that of a reference agent.
Owner:ESCARY JEAN LOUIS
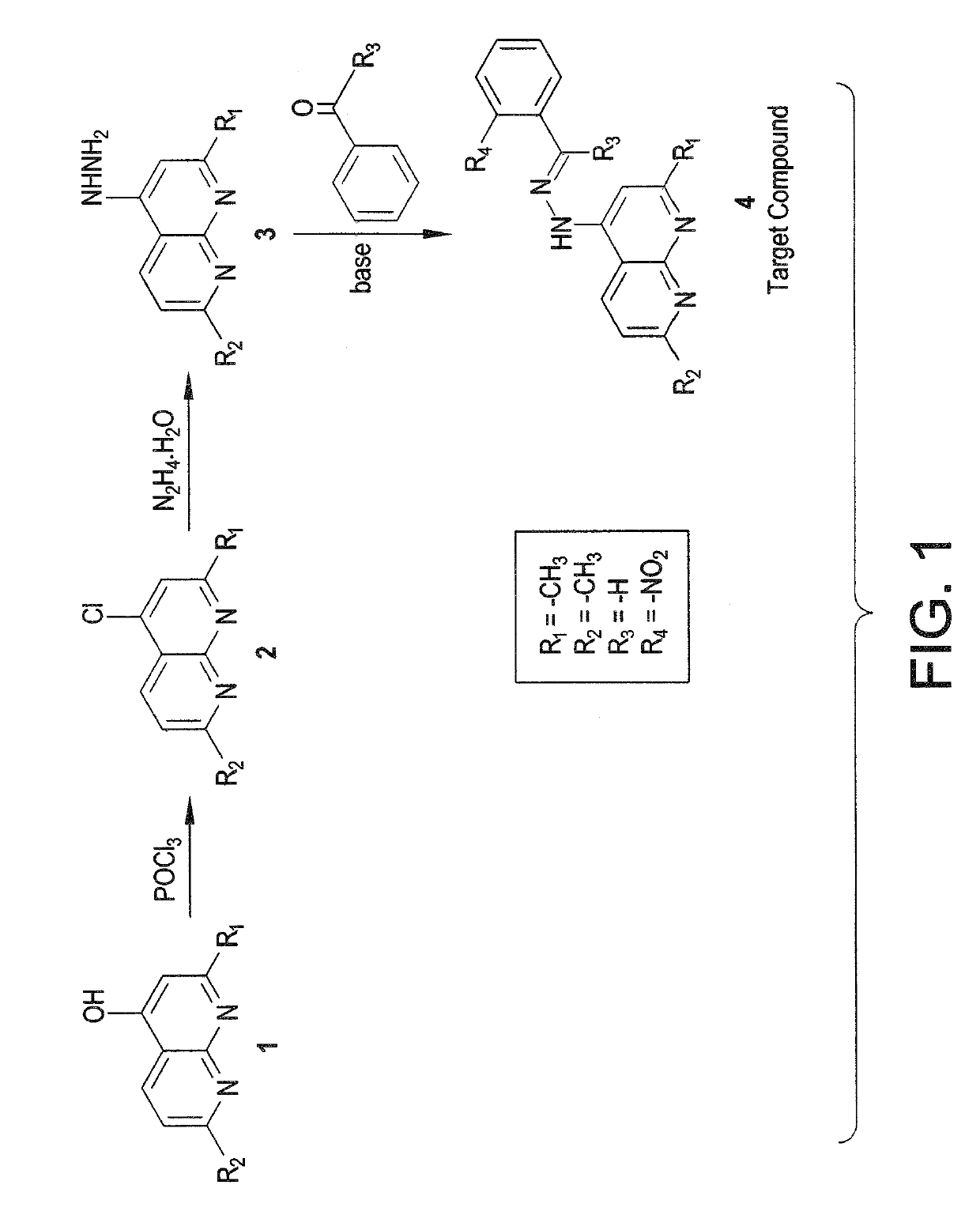
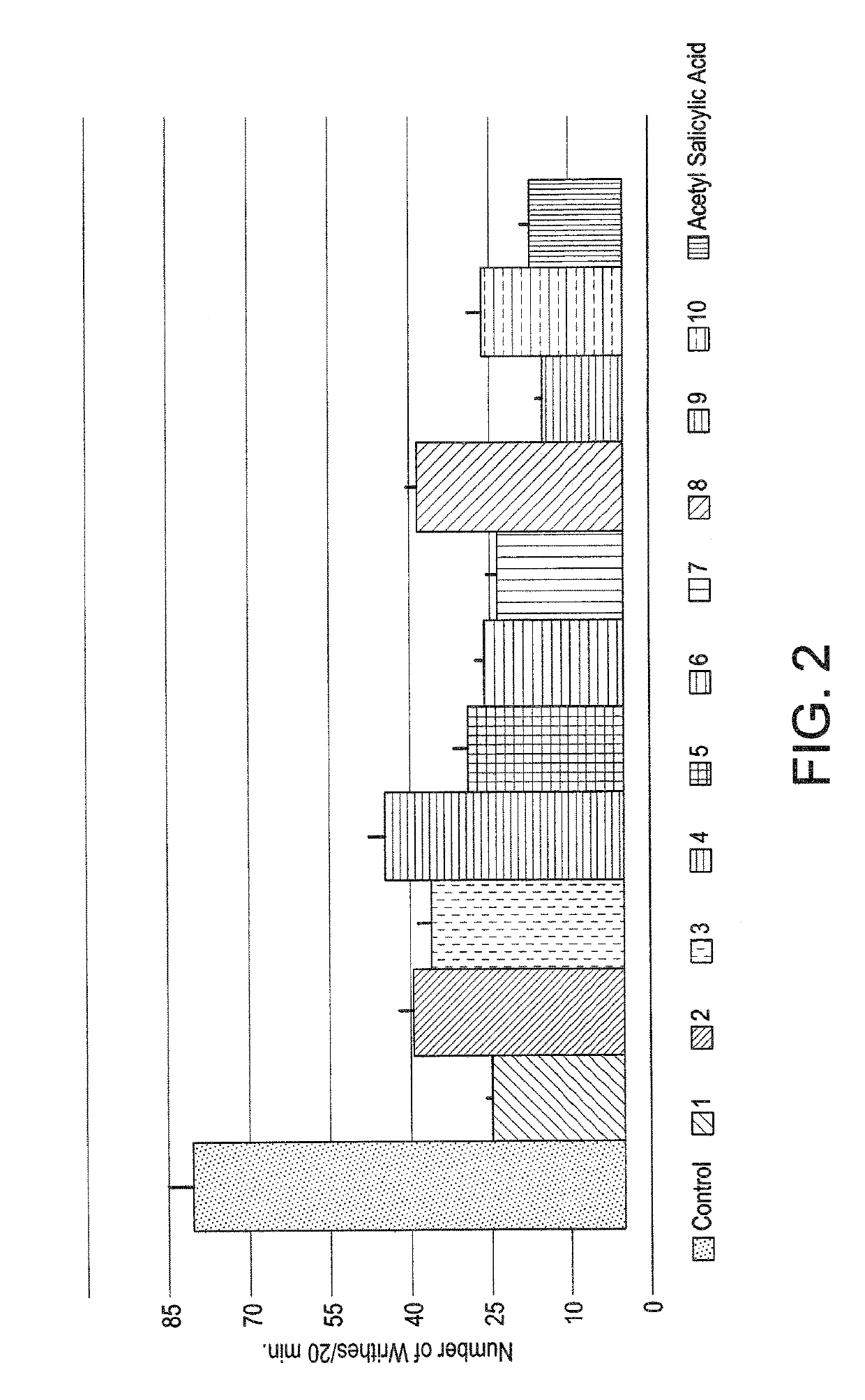
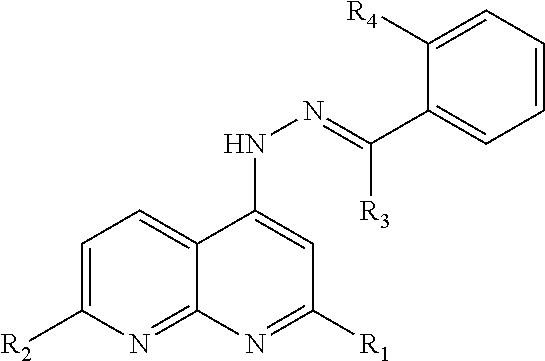
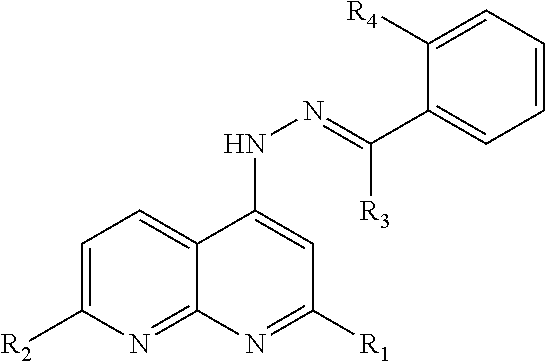
Naphthyridinyl hydrazine derivatives as potent peripheral analgesic agents
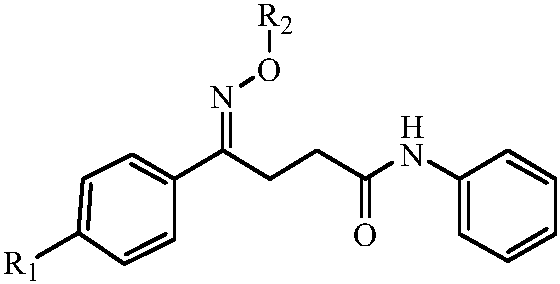
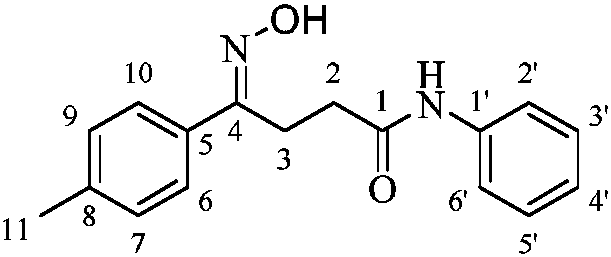
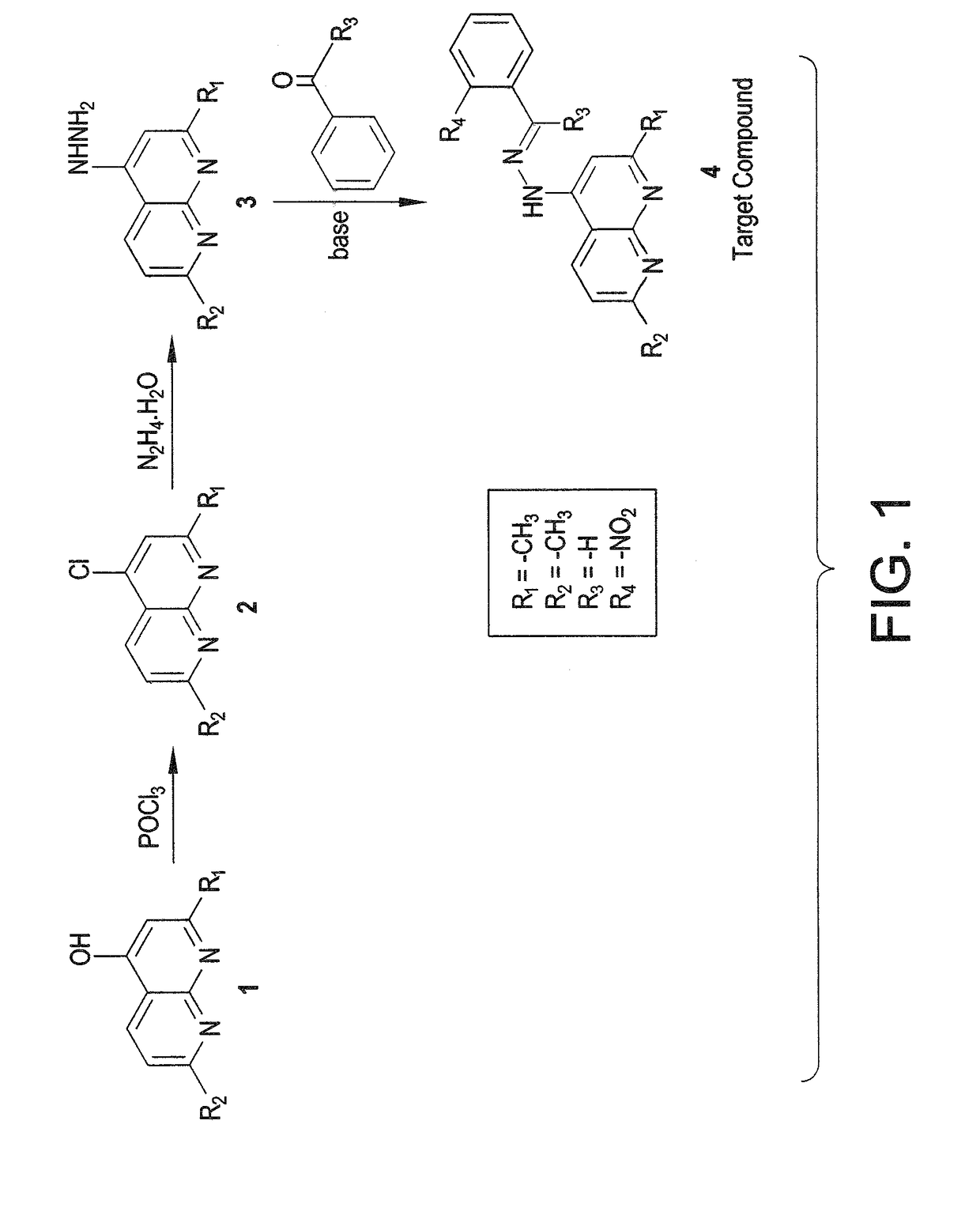
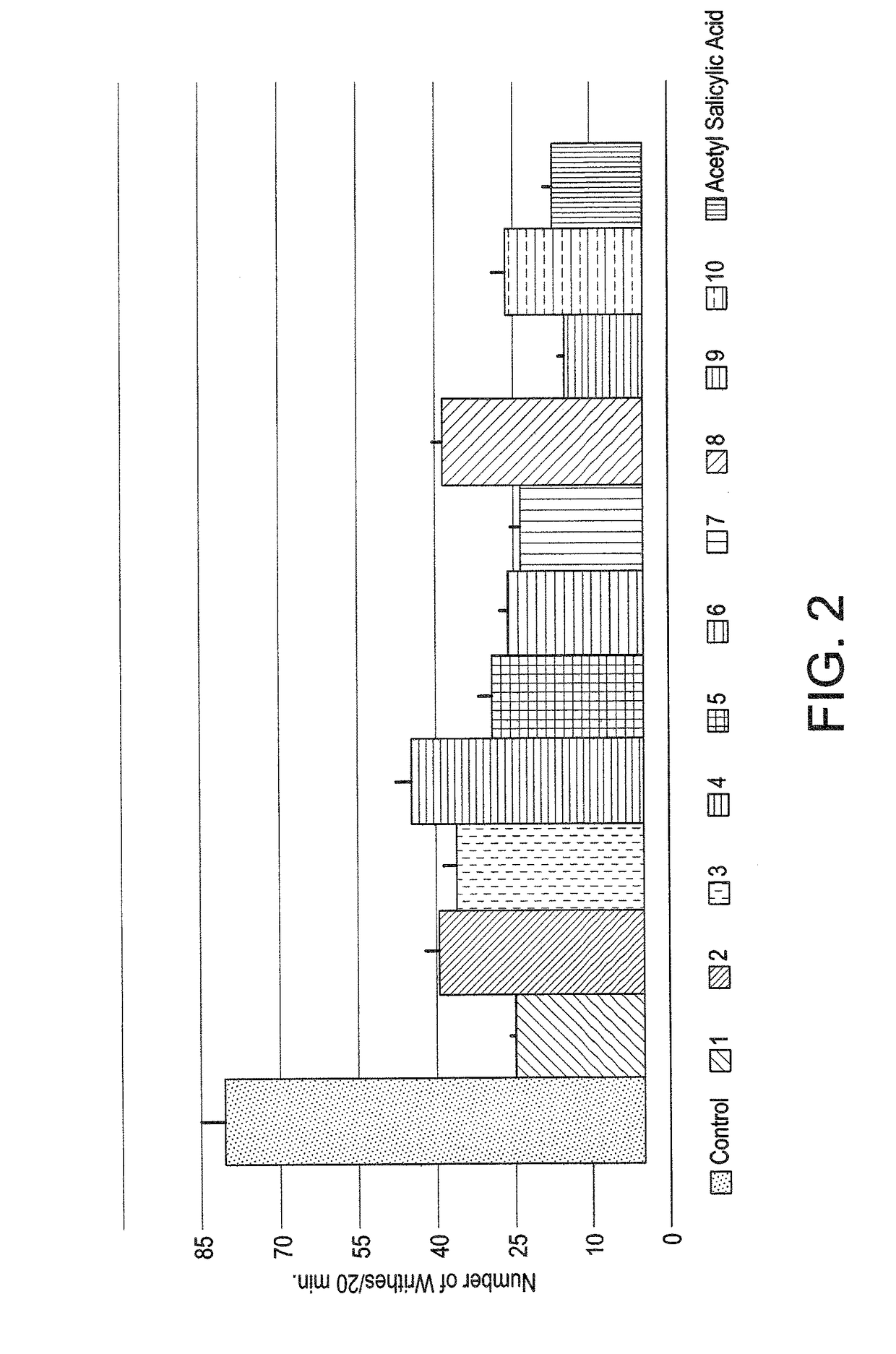
The naphthyridinyl hydrazine derivatives as potent peripheral analgesic agents are (E)-2-(substituted benzylidene)-1-(2,7-dialkyl-1,8-naphthyridinyl) hydrazines that provide effective peripheral analgesic activity, as demonstrated using the mouse writhing test. The new target compounds include at least one compound that demonstrates higher potency in providing analgesic relief in mice (Protection (%)=81.44) compared to the reference drug acetyl salicylic acid (Protection (%)=78.47). These results demonstrated that the target compound exerts acute analgesic action, suggesting that it may represent an alternative in the development of new therapeutic strategies. Preferably, the (E)-2-(substituted benzylidene)-1-(2,7-dialkyl naphthyridinyl) hydrazine has the formula:wherein R1 and R2 are alkyl, R3 is hydrogen, and R4 is NO2.
Owner:KING SAUD UNIVERSITY
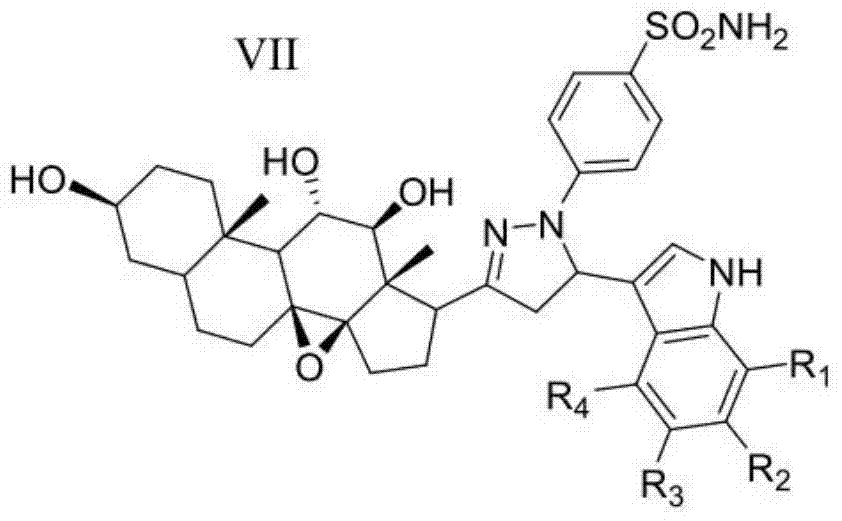
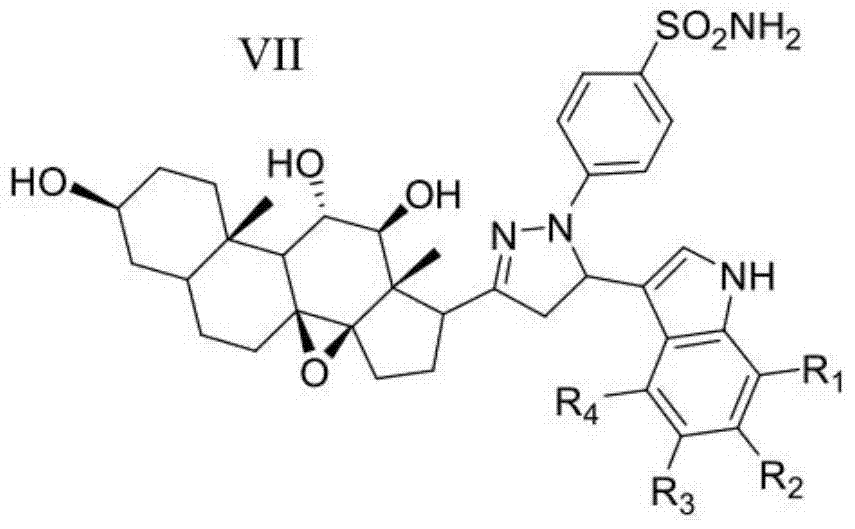
2h-pyrazole sulfanilamide steroid saponin aglycone derivative containing indole framework and preparation method and application thereof
ActiveCN104774241AExperimental repeatability is strongImprove stabilityOrganic active ingredientsSteroidsCancer cellSulfanilamide
The invention discloses a 2h-pyrazole sulfanilamide steroid saponin aglycone derivative containing indole framework and a preparation method and application thereof. The structure of the 2h-pyrazole sulfanilamide steroid saponin aglycone derivative containing the indole framework is shown as the formula VII in the specification. R1 is selected from H, CH3 and CH2CH3. R2 is selected from H, CH3, CH2CH3, F, C1 and Br. R3 is selected from H and CH3. R4 is selected from H and CH3. The 2h-pyrazole sulfanilamide steroid saponin aglycone derivative has a remarkable restraint effect on breast cancer cells (MCF-7), cervical cancer cells (HeLa), lung cancer cells (A549) and hepatoma cancer cells (HepG2) of human body, has identical or superior cell toxicity to human renal epithelial cells (293T) compared with a positive reference drug (Celecoxib), has better biological cavity, higher selectivity and lower toxicity, and can be used for preparing anti-cancer drugs.
Owner:JIANGSU NAIQUE BIOLOGICAL ENG
Application of chicoric acid in preparing drug for preventing respiratory syncytial virus
ActiveCN107050010AActive ingredients are clearOrganic active ingredientsOrganic compound preparationBULK ACTIVE INGREDIENTActive ingredient
The invention discloses a novel application of chicoric acid, namely an application of chicoric acid in preparing a drug for preventing respiratory syncytial virus (RSV). The inventor of the invention screens antiviral components in an echinacea plant by applying a traditional Chinese medicine chemical method and finds the optimum antiviral active ingredient, chicoric acid, through an in vitro antiviral pharmacological experiment and finds that the in vitro antiviral activity of chicoric acid on RSV is the strongest, and is superior to that of a reference drug, ribavirin, so that chicoric acid can be used for preparing the drug for preventing RSV as an active ingredient. In specific application, a pharmaceutically acceptable matrix or auxiliary material can be properly added to chicoric acid, and chicoric acid can be also combined with proper drugs in application.
Owner:SHANDONG UNIV OF TRADITIONAL CHINESE MEDICINE
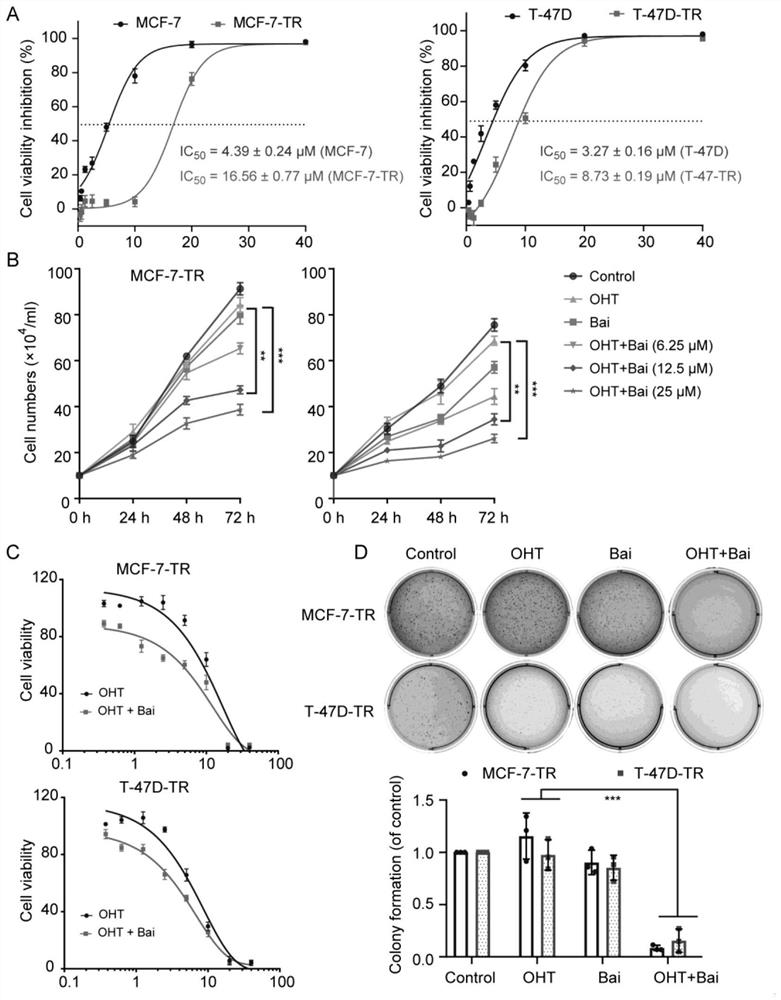
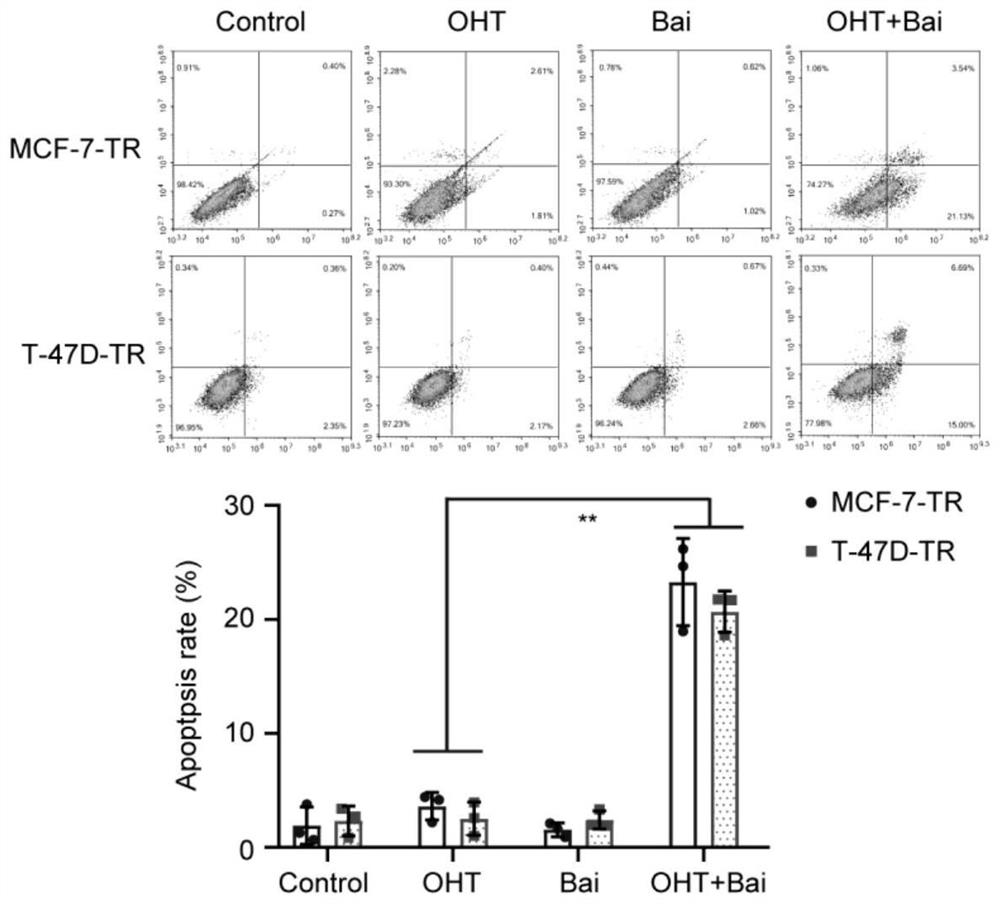
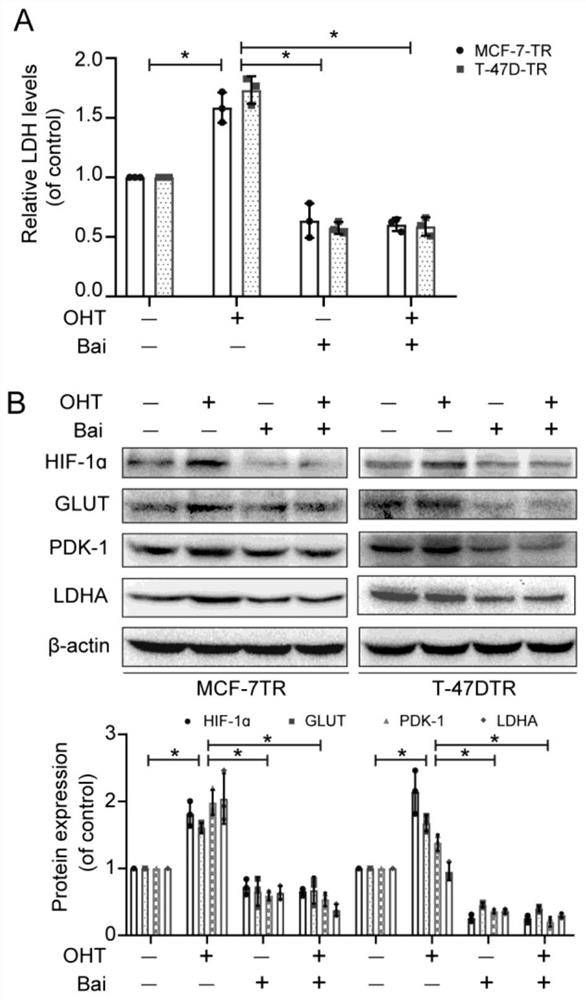
Drug-resistant breast cancer treatment drug and screening method thereof
InactiveCN113444765AEnhance cancer treatment outcomesHas a sensitizing effectOrganic active ingredientsCompound screeningOncologyCancer research
The invention relates to a drug-resistant breast cancer drug screening method and an obtained effective treatment drug. The drug-resistant breast cancer drug screening method comprises the following steps that a drug-resistant breast cancer cell strain is selected, the resistance of the cell strain to an anti-breast cancer drug is confirmed, and a reference drug is selected; and the influence effect of a to-be-screened drug on cell apoptosis inhibition of a reference drug is detected, the influence on the glycolysis level of drug-resistant cells and the influence on mitochondria of the drug-resistant cells is detected, and the screened baicalein with excellent comprehensive evaluation is an effective anti-breast cancer drug sensitizer. The drug-resistant breast cancer drug screening method is especially suitable for obtaining a matched sensitizing drug aiming at a specific drug-resistant drug, so that precise medical treatment aiming at a specific drug-resistant breast cancer patient is facilitated.
Owner:GUIZHOU MEDICAL UNIV
Water-soluble maslinic acid derivative as well as preparation method and application thereof
InactiveCN109232708AGood water solubilityStrong inhibitory activityMetabolism disorderSteroidsReduction ActivityReference drug
The invention relates to a water-soluble maslinic acid derivative as well as a preparation method and application thereof, which belongs to the field of medicines. According to the water-soluble maslinic acid derivative provided by the invention, a series of hydrophilic amino acids are respectively introduced on maslinic acid C-28 carboxyl sites. Discovered from an experiment result, the hydrophilic property is significantly improved, and the glucose reduction activity of N(2alpha,3beta-dihydroxyl oleanane-12-ene-28-amide)-L-aspartic acid is apparently higher than that of a reference drug acarbose.
Owner:JIANGXI AGRICULTURAL UNIVERSITY
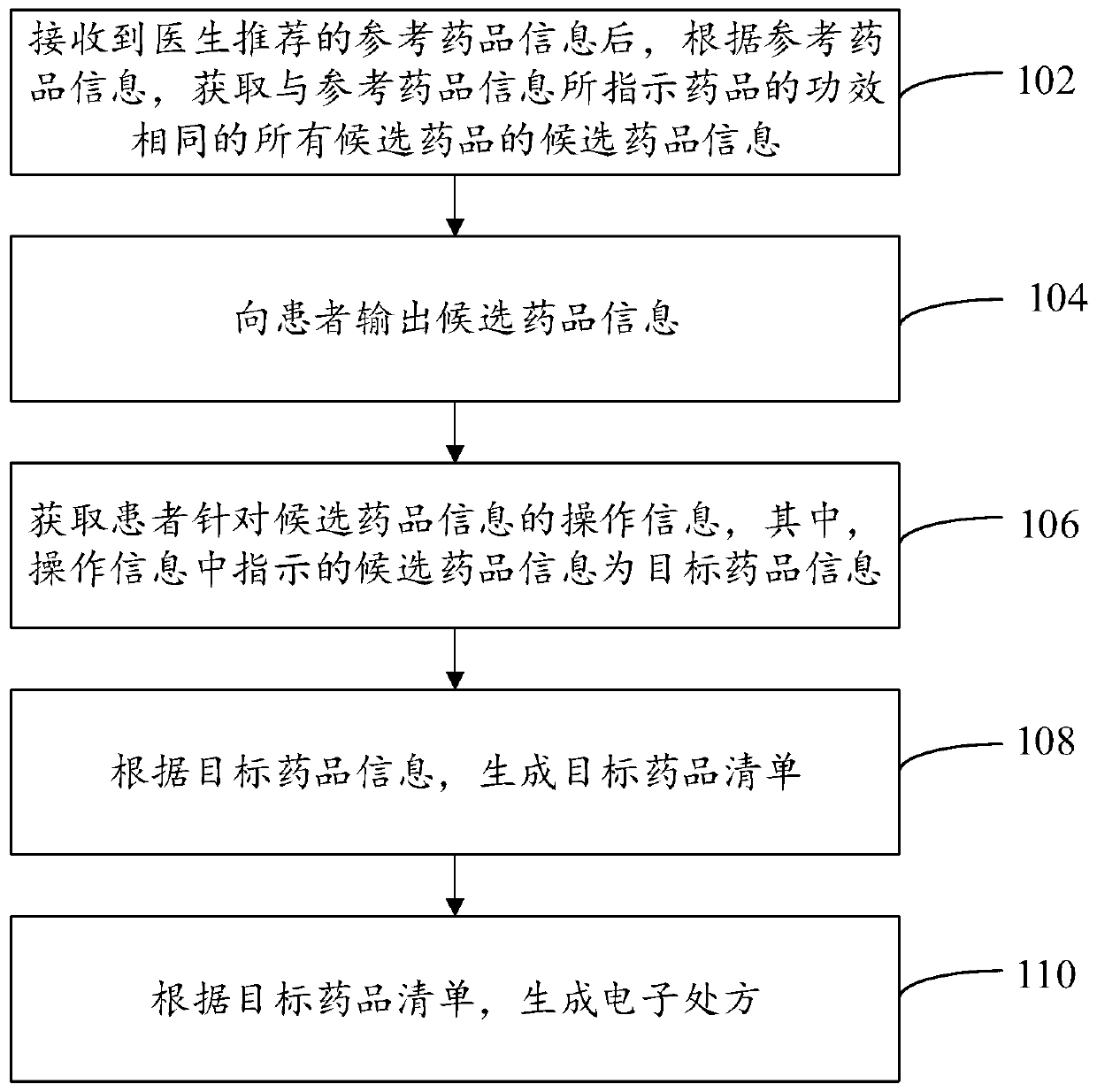
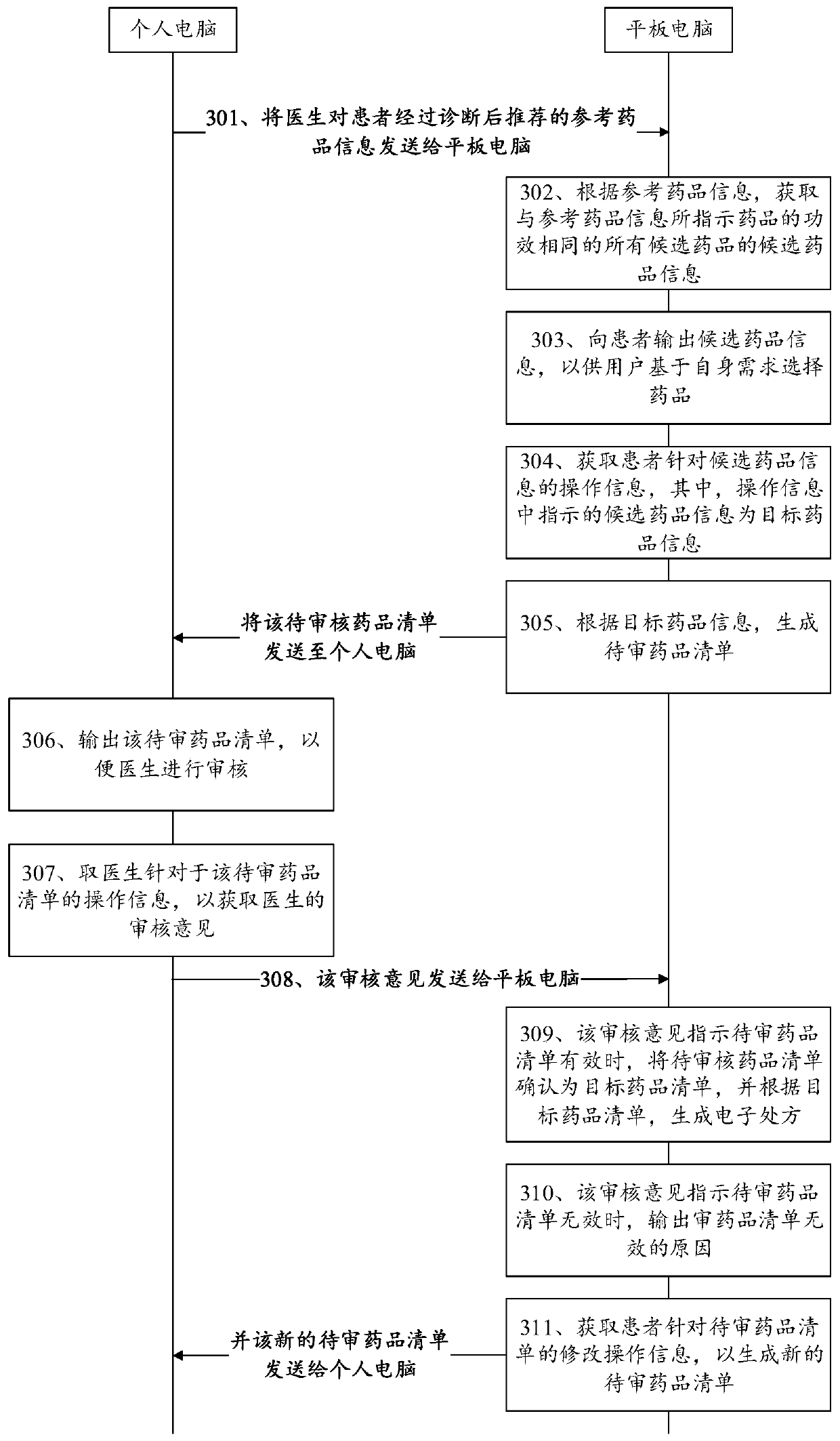
Electronic prescription generation method and device, computer device and readable storage medium
PendingCN109830273AMeet individual needsImprove doctor-patient relationshipDrug and medicationsDrug indicatedDrug product
The embodiment of the invention provides an electronic prescription generation method and device, a computer device and a readable storage medium, and relates to the technical field of computers. Theelectronic prescription generation method includes the steps: after receiving reference drug information recommended by a doctor, obtaining candidate drug information of all the candidate drugs havingthe same efficacy as the drug indicated by the reference drug information according to the reference drug information; outputting the candidate drug information to a patient; obtaining operation information of the patient for the candidate drug information, wherein the candidate drug information indicated in the operation information is the target drug information; generating a target drug list according to the target drug information; and further, generating an electronic prescription according to the target drug list. Therefore, for the technical scheme of the electronic prescription generation method, during the overall process of generation of an electronic prescription, both the patient and the doctor participate in the selection of the drug, so that different electronic prescriptions can be formulated for different patients to meet the individual needs of different patients.
Owner:平安万家医疗管理有限责任公司
Use of a solid fraction sensor to evaluate a solid fraction of a target pharmaceutical sample and solid fraction sensor
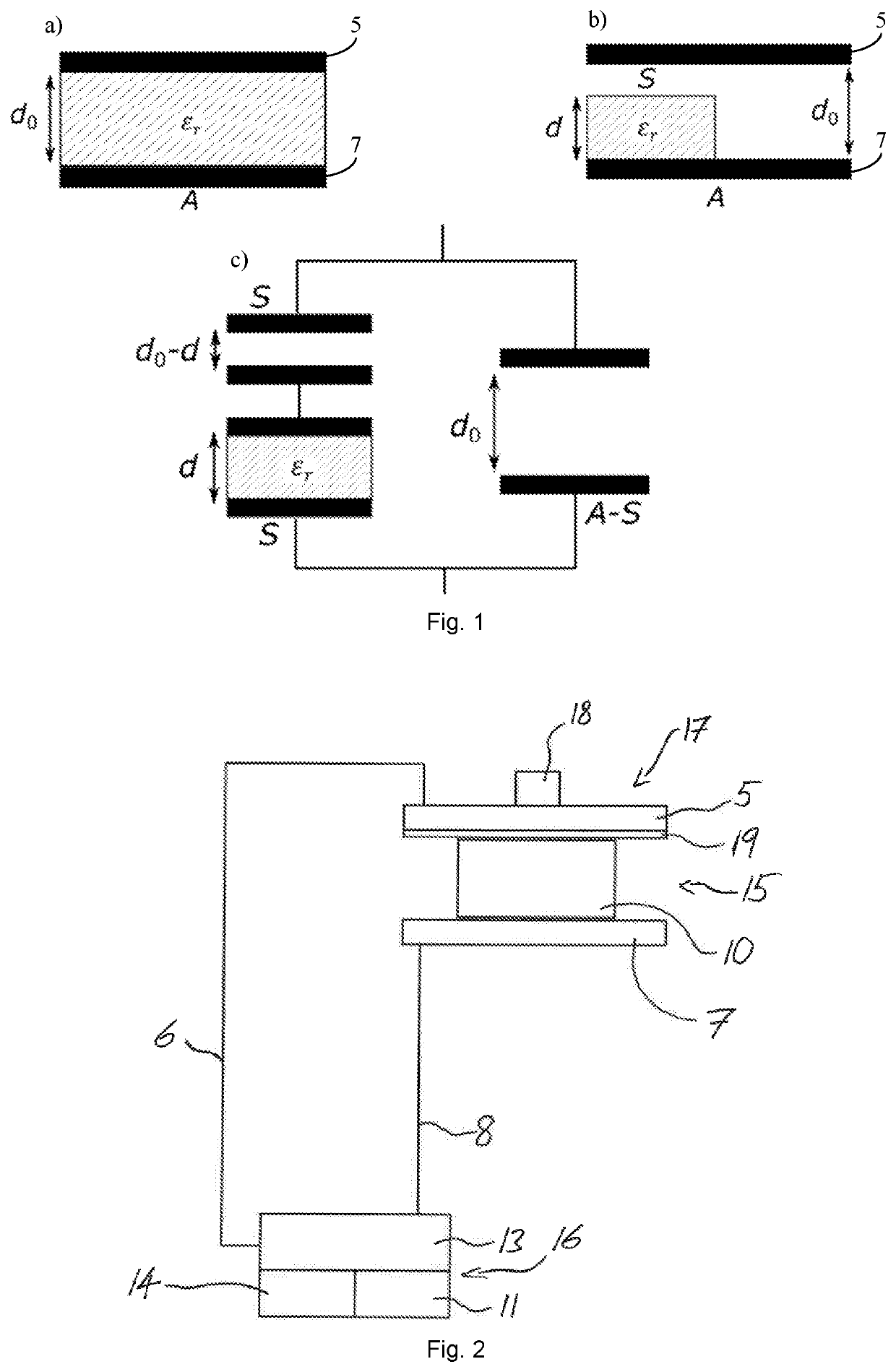
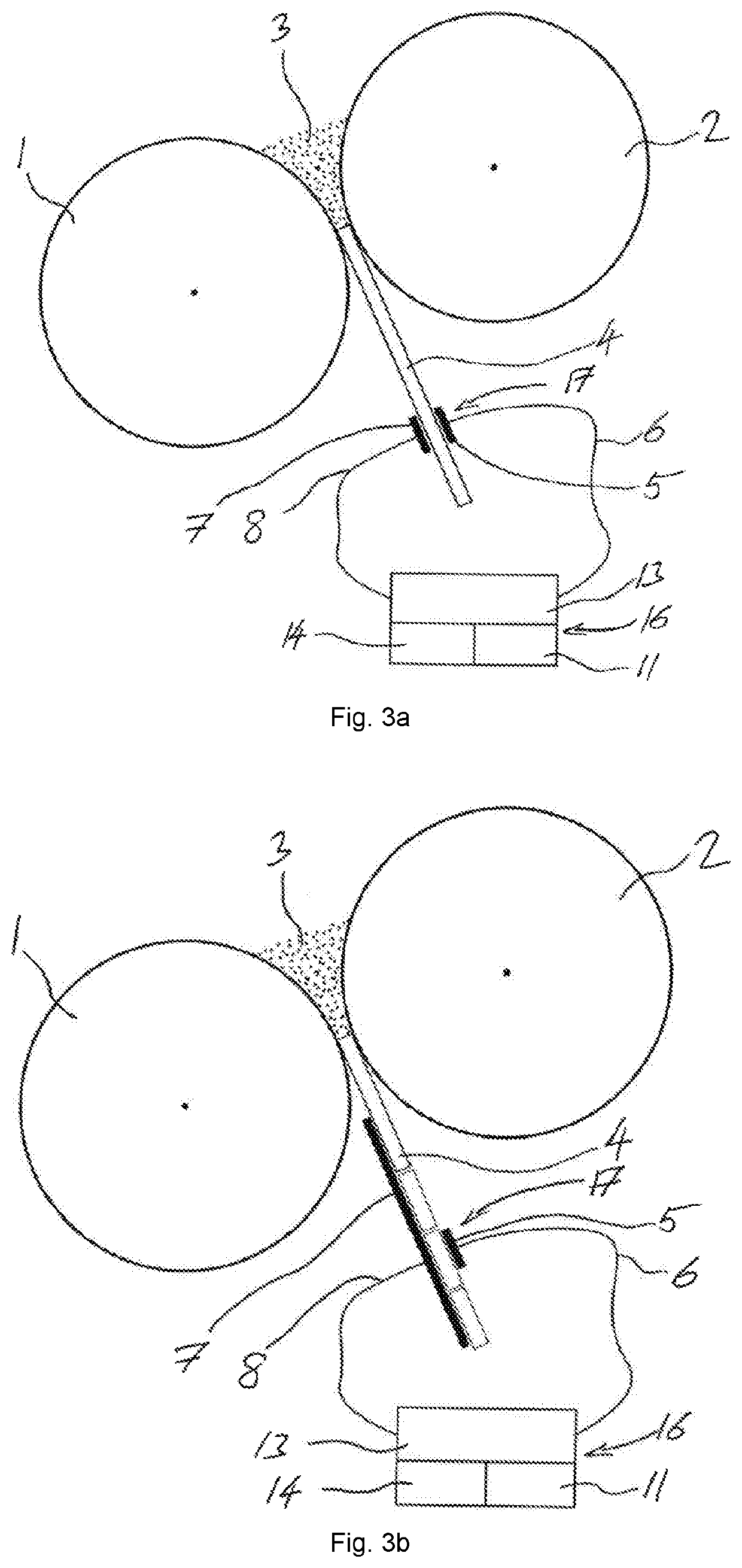
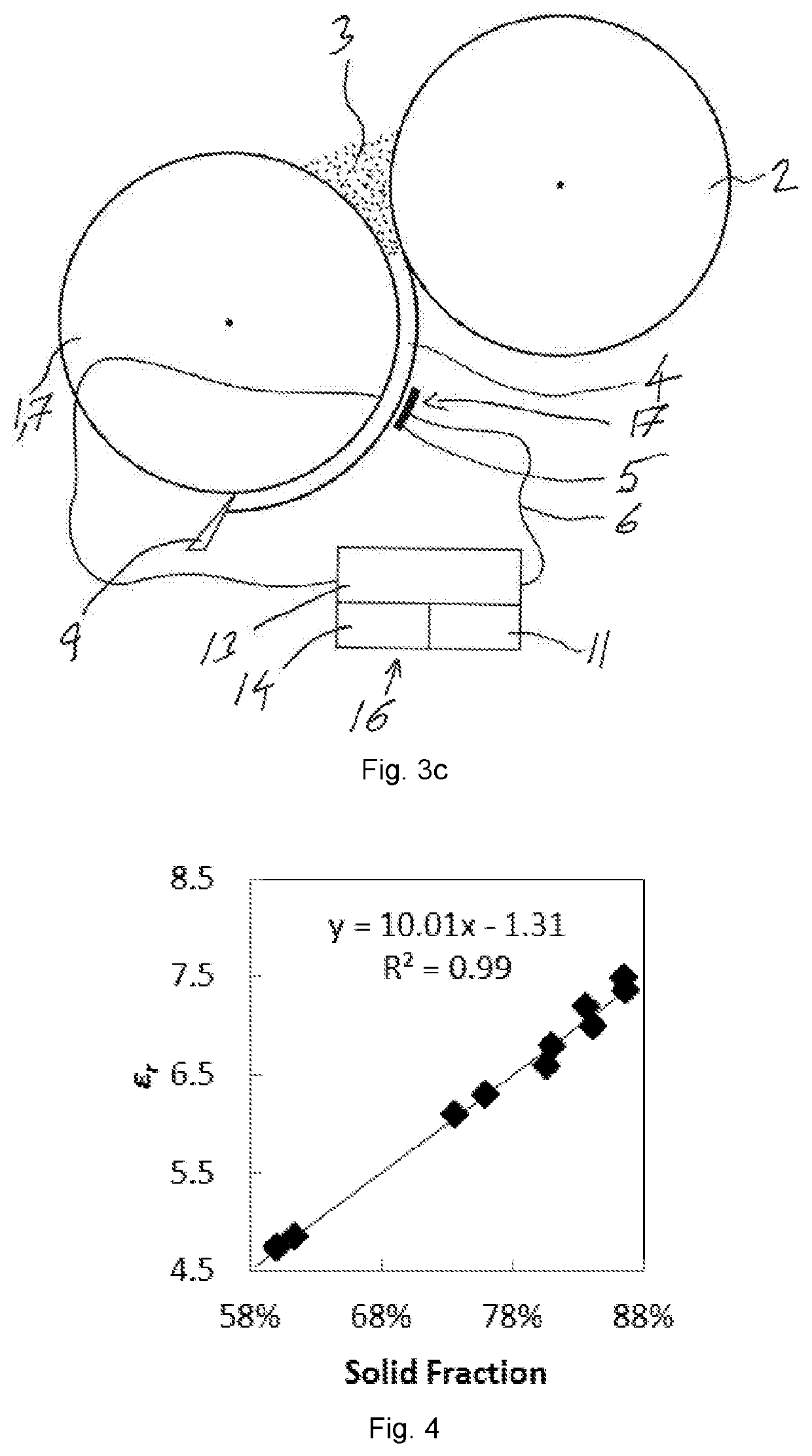
ActiveUS20200217811A1Efficient and accurate assessmentMeasurement can be reduced and minimizedMaterial analysis by optical meansContainer/cavity capacity measurementCapacitanceElectrical conductor
For the use of a solid fraction sensor (17) to evaluate a solid fraction of a target pharmaceutical sample (4, 10) the solid fraction sensor (17) has a first conductor element (5), a second conductor element (7), an operation space (15) and an energy source (13) arranged to generate an electric field in the operation space (15) by means of the first conductor element (5) and the second conductor element (7), comprising positioning the target pharmaceutical sample (4, 10) in the operation space (15) of the solid fraction sensor (17), determining a capacitance between the first and second conductor element (5, 7) with the target pharmaceutical sample (4, 10) located in the operation space (15), and converting the determined capacitance together with information about a composition of a reference pharmaceutical sample having the essentially same dielectric properties as the target pharmaceutical sample (4, 10) and about a thickness of the reference pharmaceutical sample (4, 10) into a solid fraction of the target pharmaceutical sample (4, 10).
Owner:F HOFFMANN LA ROCHE & CO AG
Analyzing High Dimensional Data Based on Hypothesis Testing for Assessing the Similarity between Complex Organic Molecules Using Mass Spectrometry
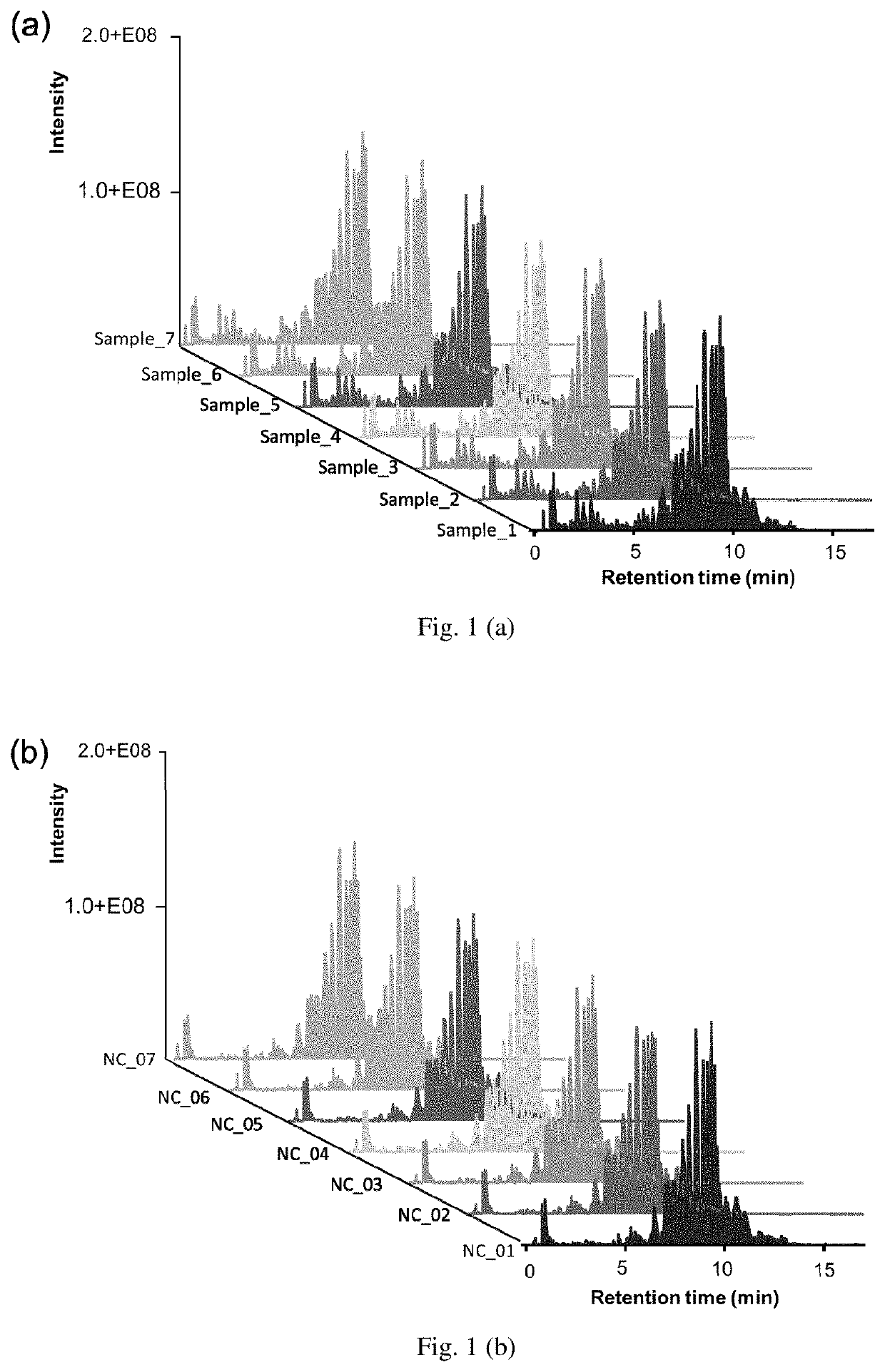
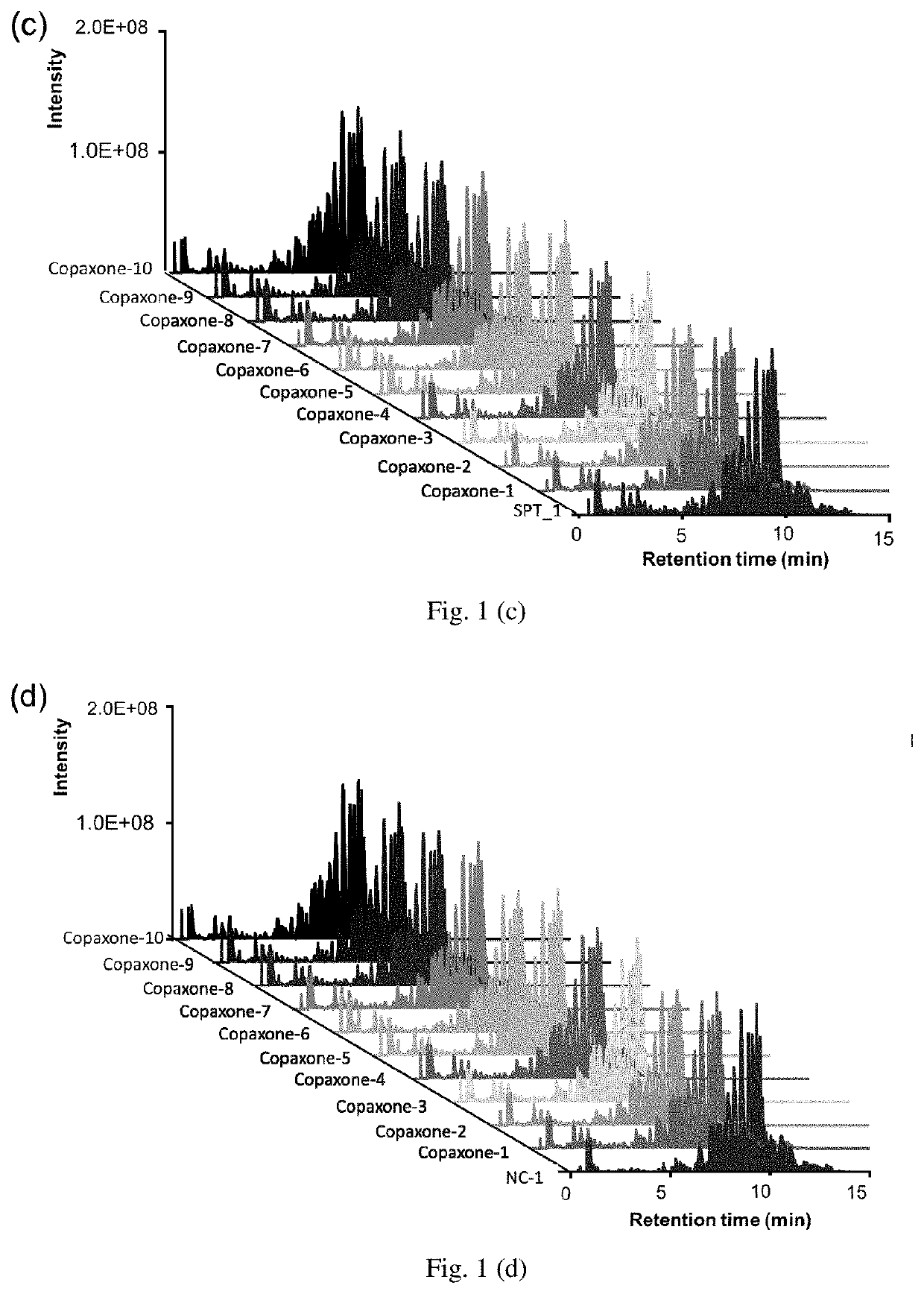
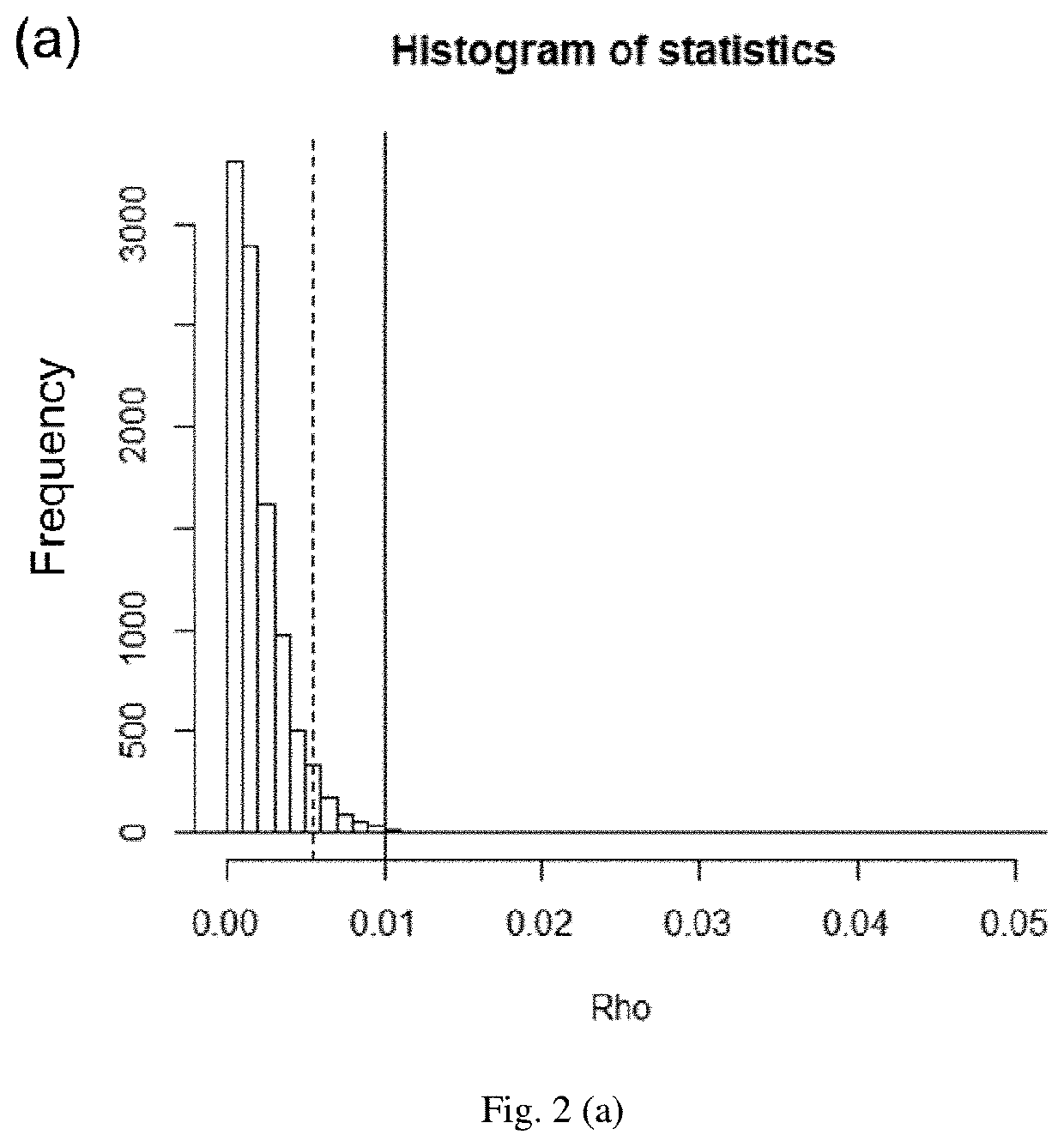
InactiveUS20200075128A1Robust resultMolecular entity identificationBiostatisticsMass Spectrometry-Mass SpectrometryPharmaceutical drug
The present invention developed a hypothesis testing approach to analyze the high-dimensional LC-MS data to assess the extent of similarity between a reference drug and generics.
Owner:SCINOPHARM TAIWAN LTD
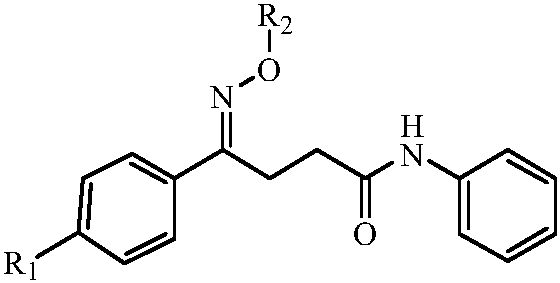
N-phenyl-4-phenylbutyramide oxime and its derivatives with anti-hepatitis B virus activity
The invention discloses N-phenyl-4-phenyl butyrylamide oxime with anti-hepatitis B virus activity and a derivative thereof. The structure of the N-phenyl-4-phenyl butyrylamide oxime is represented by the following general formula: shown in the specification. An in-vitro HepG2.2.15 cell activity experiment shows that the N-phenyl-4-phenyl butyrylamide oxime has a certain inhibition effect on HBV surface antigens (HBsAg) and e antigens (HBeAg). Under the same condition, the inhibition rates of positive reference drug Lamivudine to HBsAg and HBeAg are respectively 50.9 percent and 45.2 percent, while the compound disclosed by the invention has the inhibition rates to HBsAg and HBeAg of 91.7 percent and 59.5 percent. The structure of the novel N-phenyl-4-phenyl butyrylamide oxime is different from that of a nucleoside similar drug, so that the N-phenyl-4-phenyl butyrylamide oxime is obvious in inhibition to the HBV activity, low in toxicity and expected to be further developed into a novel anti-hepatitis B virus drug.
Owner:GUANGXI UNIV
Naphthyridinyl hydrazine derivatives as potent peripheral analgesic agents
InactiveUS20180362524A1Improve performanceEffective analgesic activityOrganic chemistryHydrogenHydrazine compound
The naphthyridinyl hydrazine derivatives as potent peripheral analgesic agents are (E)-2-(substituted benzylidene)-1-(2,7-dialkyl-1,8-naphthyridinyl) hydrazines that provide effective peripheral analgesic activity, as demonstrated using the mouse writhing test. The new target compounds include at least one compound that demonstrates higher potency in providing analgesic relief in mice (Protection (%)=81.44) compared to the reference drug acetyl salicylic acid (Protection (%)=78.47). These results demonstrated that the target compound exerts acute analgesic action, suggesting that it may represent an alternative in the development of new therapeutic strategies. Preferably, the (E)-2-(substituted benzylidene)-1-(2,7-dialkyl naphthyridinyl) hydrazine has the formula:wherein R1 and R2 are alkyl, R3 is hydrogen, and R4 is NO2.
Owner:KING SAUD UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com