Compound with antitumor activity and preparation method and application of compound
Anti-tumor activity, anti-tumor drug technology, applied in the application of the drug in anti-tumor, anti-tumor drug and its preparation, 3′,4′-dimethoxychalcone derivatives and its preparation field , can solve the problem of no anti-tumor activity, and achieve the effects of avoiding methoxyl damage, reducing synthesis cost, and reducing dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
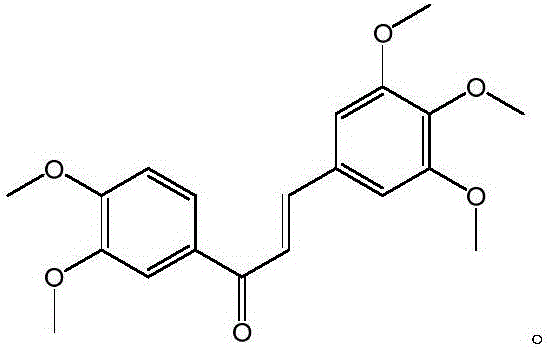
[0017] Example 1 Preparation of 3,4,5-trimethoxy-3',4'-dimethoxychalcone
[0018]
[0019] Concrete preparation steps are as follows:
[0020] Take 0.906g of 3,4-dimethoxyacetophenone and 0.976g of 3,4,5-trimethoxybenzaldehyde, dissolve in 40mL of absolute ethanol, add dropwise 1.5mL of 50% NaOH solution, and react for 48h. The pH of the reaction solution was adjusted to neutral with dilute hydrochloric acid, and 1 times the amount of water was added to precipitate a precipitate, which was filtered by suction to obtain a yellow solid, which was washed with a small amount of water. After drying and weighing, 0.795 g of light yellow 3,4,5-trimethoxy-3',4'-dimethoxychalcone solid was obtained. The yield was 44.3%. The synthetic route is as follows:
[0021]
[0022] The hydrogen spectrum of 3,4,5-trimethoxy-3',4'-dimethoxychalcone is as follows figure 1 Shown: 1 H NMR (400MHz, CDCl 3 )δ7.71(t, J=13.4Hz, 2H, CH-9, CH-5), 7.63(s, 1H, CH-1), 7.43(d, J=15.5Hz, 1H, CH-8), ...
Embodiment 2
[0023] Example 2 Determination of the activity of 3,4,5-trimethoxy-3',4'-dimethoxychalcone in anti-three tumor cells
[0024] 1 Preparation of solution:
[0025] Preparation of 10% FBS culture medium: purchase HyClone RPMI Medium Modified 1640 medium, 500mL per bottle, add 10% fetal bovine serum and 1% double antibody solution, the preparation of the medium is carried out on the ultra-clean workbench, and then placed Store in refrigerator at 4°C.
[0026] Preparation of PBS buffer solution: PBS phosphate buffer solution powder, add deionized water, fully dissolve, and store in refrigerator at 4°C after autoclaving.
[0027] Preparation of MTT solution: MTT dry powder 0.25g, dissolved in 50mL PBS buffer, sterilized by filtration with 0.22uM membrane, and stored in a refrigerator at -12°C.
[0028] Preparation method of drug mother solution: dissolve the compound (3,4,5-trimethoxy-3',4'-dimethoxychalcone) provided in Example 1 of the present invention in 40uL DMSO, add 2mL of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com