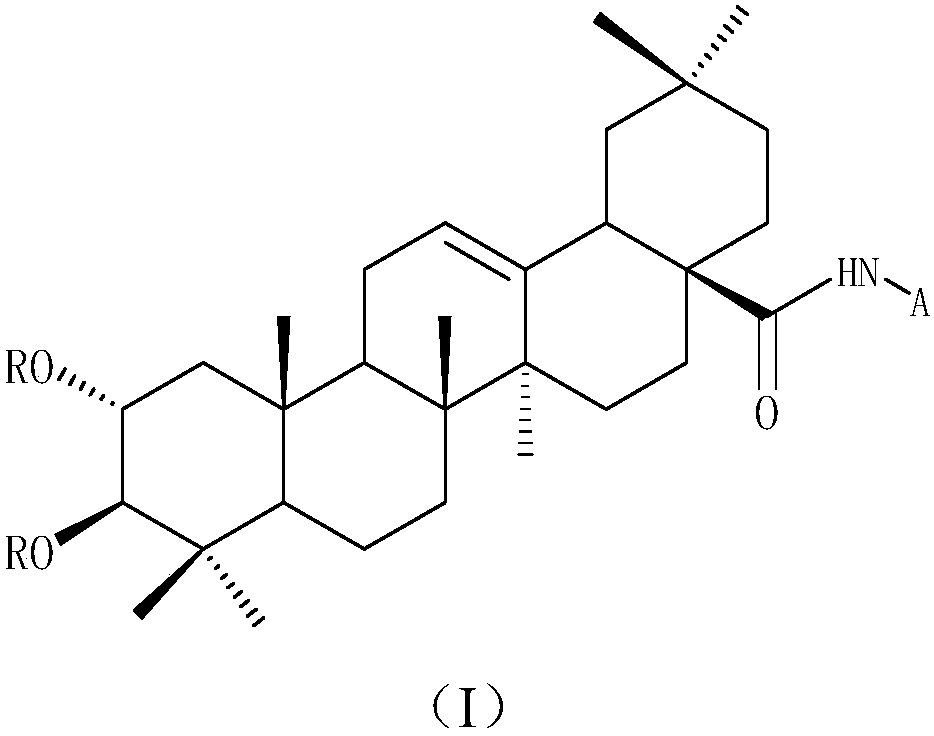
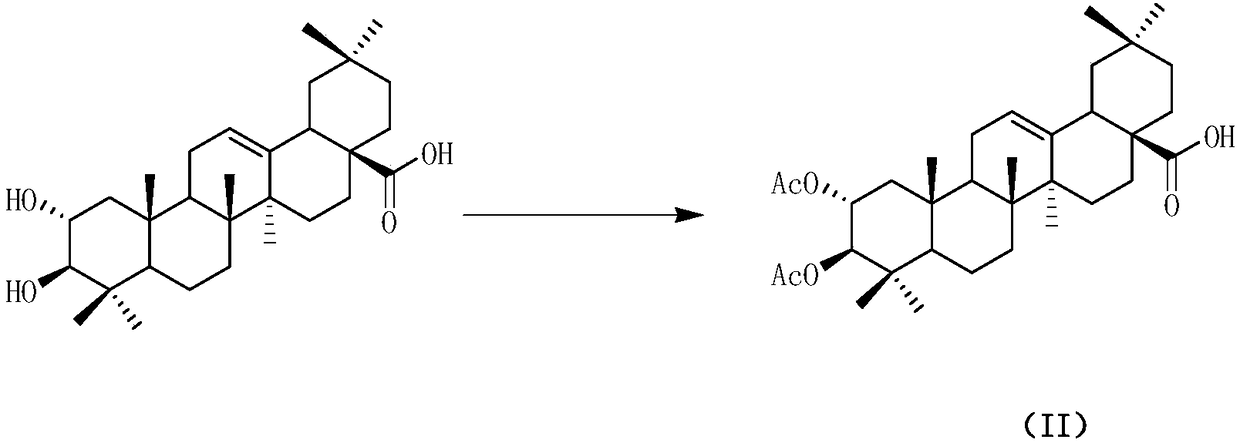
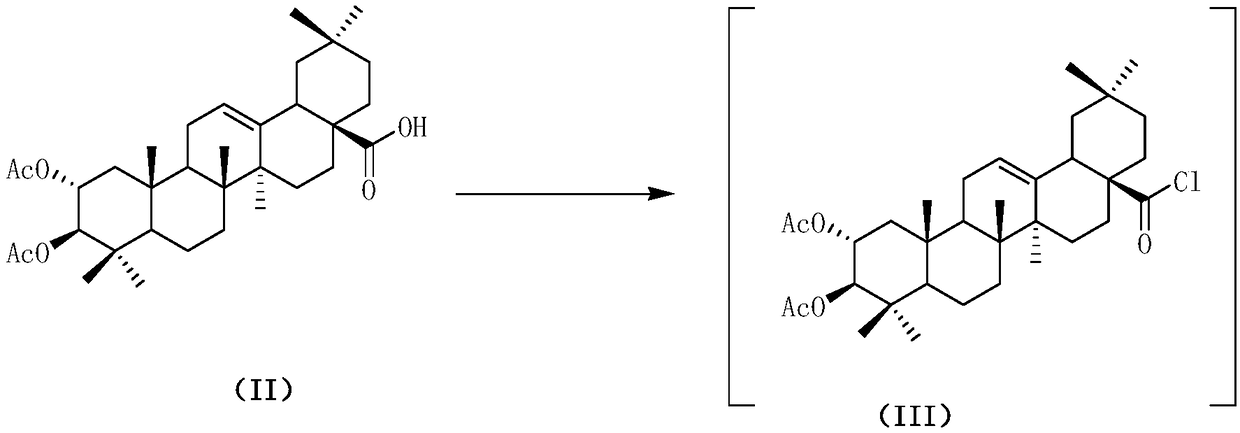
Water-soluble maslinic acid derivative as well as preparation method and application thereof
A technology of maslinic acid and derivatives, which is applied in the field of medicine, can solve the problems of poor water solubility and low bioavailability, and achieve the effect of inhibiting activity and improving water solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] (1) Synthesis of 2α,3β-diacetoxyoleanane-12-ene-28-carboxylic acid 2
[0051] Dissolve maslinic acid (0.3g, 0.63mmol) in 10ml of pyridine, slowly add 7.5ml of acetic anhydride dropwise under ice bath, and react for 24h in the bathroom after dropping the ice. After the reaction, the reaction solution was washed with 1N hydrochloric acid 50ml*3, and the organic layer was separated with saturated NaHCO 3 and distilled water, dried over anhydrous sodium sulfate, filtered, concentrated by rotary evaporation, and then subjected to silica gel column chromatography (petroleum ether: ethyl acetate = 4:1) to obtain a white solid 1 (yield 93.2%). Mp: 228~233℃. 1 H NMR (400MHz, CDCl 3 ): δ5.26(t, J=12.0Hz, 1H), 5.11-5.07(m, 1H), 4.75(d, J=12.0Hz, 1H), 2.82(dd, J=6.0, 6.0Hz, 1H) ,2.06(d,J=12.0Hz,3H),2.02-1.99(m,1H),1.98(s,3H),1.95-1.90(m,1H),1.87-1.82(m,1H),1.79-1.73 (m,1H),1.71-1.67(m,1H),1.63-1.61(m,1H),1.60(s,1H),1.58-1.57(d,J=12.0Hz,1H),1.56-1.54(m ,1H),1.48-1.45(m,1H),1.44...
Embodiment 2
[0059] (1) Synthesis of N-(2α,3β-diacetoxyoleanane-12-en-28-amide)-L-isoleucine methyl ester 4b
[0060] Referring to the method of Example 1, a white solid 4b was obtained from L-isoleucine methyl ester hydrochloride and intermediate 3 (yield 82.4%). Mp:117-136℃. 1 H NMR (600MHz, CDCl 3 ):δ6.44(d,J=6.0Hz,1H),5.42(t,J=6.0Hz,1H),5.11-5.06(m,1H),4.75(d,J=12.0Hz,1H),4.50 -4.48(m,1H),3.69(s,3H),2.65(dd,J=6.0,3.6Hz,1H),2.04(s,3H),2.03-1.98(m,2H),1.97(s,3H ),1.96-1.93(m,1H),1.89-1.84(m,2H),1.77(t,J=24.0Hz,1H),1.65-1.52(m,8H),1.48-1.44(m,2H), 1.41-1.34(m,2H),1.29-1.27(m,1H),1.21-1.17(m,3H),1.14(s,3H),1.04(s,3H),0.94(t,J=12.0Hz, 3H),0.91(s,6H),0.89(d,J=3.6Hz,6H),0.87(d,J=12.0Hz,3H),0.68(s,3H).
[0061] (2) Synthesis of N-(2α,3β-dihydroxyoleanane-12-en-28-amide)-L-isoleucine 5b
[0062] Referring to the method of Example 1, 4b was hydrolyzed to obtain white solid 5b (65.4% yield). Mp:147-184℃. 1 HNMR (600MHz, DMSO-d 6 ):δ6.99(d,J=6.0Hz,1H),5.23(t,J=12.0Hz,1H),4.08(t,J=12.0Hz,1...
Embodiment 3
[0064] (1) Synthesis of N-(2α,3β-diacetoxyoleanane-12-ene-28-amide)-L-valine methyl ester 4c
[0065] Referring to the method of Example 1, a white solid 4c was obtained with L-valine methyl ester hydrochloride and intermediate 3 (85.6% yield). Mp:168-180℃. 1 H NMR (600MHz, CDCl 3):δ6.40(d,J=6.0Hz,1H),5.43(t,J=6.0Hz,1H),5.11-5.06(m,1H),4.75(d,J=12.0Hz,1H),4.45 -4.43(m,1H),3.70(s,3H),2.66(dd,J=6.0,3.6Hz,1H),2.13-2.06(m,1H),2.05(s,3H),2.03-2.00(m ,2H),1.98(s,3H),1.97-1.93(m,1H),1.90-1.85(m,1H),1.77-1.73(m,J=24.0Hz,1H),1.70(s,1H), 1.66-1.63(m,2H),1.61-1.58(m,2H),1.56-1.52(m,1H),1.49-1.44(m,1H),1.42-1.33(m,2H),1.29-1.27(m ,1H),1.25(s,3H),1.21-1.18(m,2H),1.14(s,3H),1.08(d,J=12.0Hz,1H),1.04(s,3H),0.97-0.95( m,1H),0.93-0.89(m,15H),0.67(s,3H).
[0066] (2) Synthesis of N-(2α,3β-dihydroxyoleanane-12-en-28-amide)-L-valine 5c
[0067] Referring to the method of Example 1, 4c was hydrolyzed to obtain white solid 5c (68.4% yield). Mp:112-128℃. 1 HNMR (600MHz, DMSO-d 6 ): δ7.23(d,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com