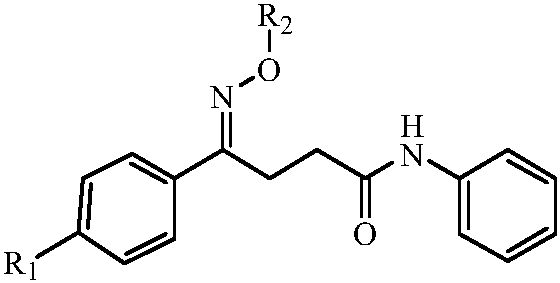
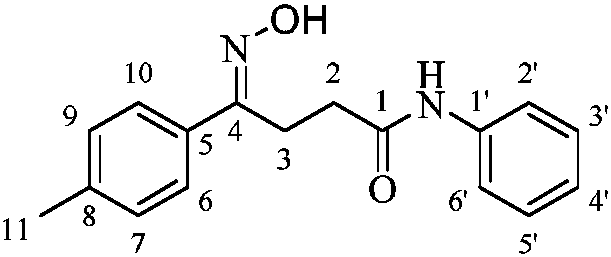
N-phenyl-4-phenylbutyramide oxime and its derivatives with anti-hepatitis B virus activity
A phenylbutyramide oxime, anti-hepatitis B technology, applied in antiviral agents, organic chemistry, drug combination, etc., can solve the problems of drug resistance, expensive interferon, synthetic interference, etc., and achieve the effect of low toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] The preparation of the first compound of embodiment 1
[0032] (1) Weigh 10mmol of toluene in a 250mL single-necked round bottom flask, add 20mL of dichloromethane, and insert 2dry tube, add 20mmol anhydrous AlCl in batches under ice-bath condition 3 After adding 0.5h, continue to stir in the ice bath for 20min, then stir overnight at room temperature. According to TLC detection, after the raw materials were completely reacted, dilute glacial hydrochloric acid was added to stop the reaction (pH2 O was recrystallized to give 4-oxo-4-(4-methylphenyl)butanoic acid.
[0033] (2) Weigh 10mmol of 4-oxo-4-(4-methylphenyl)butyric acid prepared in step (1) of the example, 10mmol of aniline, and 5% 4-dimethylaminopyridine in a 100mL single-port round bottom flask, add 20mL tetrahydrofuran, loaded with CaCl 2 Stir in an ice bath for 10 minutes, add 11 mmol of dicyclohexylcarbodiimide in batches, complete the addition after 20 minutes, continue to stir in an ice bath for 0.5 h, ...
Embodiment 2
[0041] The preparation of the second compound of embodiment 2
[0042] (1) Same as step (1) of Example 1.
[0043] (2) Same as step (2) of Example 1.
[0044] (3) Weigh 10mmol of the N-phenyl-4-oxo-4-(4-methylphenyl)butyramide prepared in the embodiment step (2), 12mmol of methoxyamine hydrochloride, and 12mmol of pyridine in a 100mL round Add 20mL of dichloromethane to the bottom flask, stir the reaction at 70°C, TLC detects that there is no raw material point after 6h, stop the reaction. The solvent was distilled off under reduced pressure, 20 mL of water was added, extracted with ethyl acetate (3×20 mL), the organic layers were combined, washed with water (3×20 mL), saturated brine (3×20 mL) successively, dried over anhydrous sodium sulfate, and reduced Concentrated under reduced pressure, separated by silica gel column chromatography, the mobile phase is composed of: petroleum ether (boiling point 60-90 ° C): ethyl acetate: dichloromethane volume ratio of 12: 2: 1 solven...
Embodiment 3
[0051] The preparation of the third compound of embodiment 3
[0052] (1) Same as step (1) of Example 1.
[0053] (2) Same as step (2) of Example 1.
[0054] (3) Weigh 10mmol of the N-phenyl-4-oxo-4-(4-methylphenyl)butyramide prepared in the embodiment step (2), 12mmol of benzyloxyamine hydrochloride, and 12mmol of pyridine in a 100mL round Add 20mL of dichloromethane to the bottom flask, stir the reaction at 70°C, TLC detects that there is no raw material point after 6h, stop the reaction. The solvent was distilled off under reduced pressure, 20 mL of water was added, extracted with ethyl acetate (3×20 mL), the organic layers were combined, washed with water (3×20 mL), saturated brine (3×20 mL) successively, dried over anhydrous sodium sulfate, and reduced Concentrated under reduced pressure, separated by silica gel column chromatography, the mobile phase is composed of: petroleum ether (boiling point 60-90 ° C): ethyl acetate: dichloromethane volume ratio of 12: 2: 1 solve...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com