Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
67 results about "Nitrobenzoates" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Benzoic acid or benzoic acid esters substituted with one or more nitro groups.
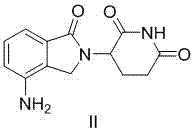
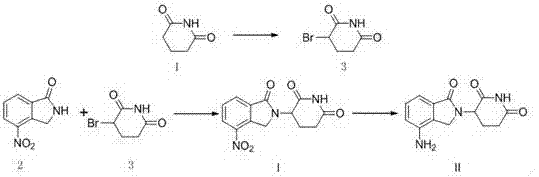
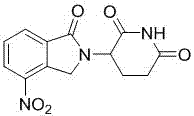
Method for preparing lenalidomide
InactiveCN104311536ASignificant technological progressOptimize the process routeCarbamic acid derivatives preparationOrganic compound preparationDicarbonateL-Glutamin
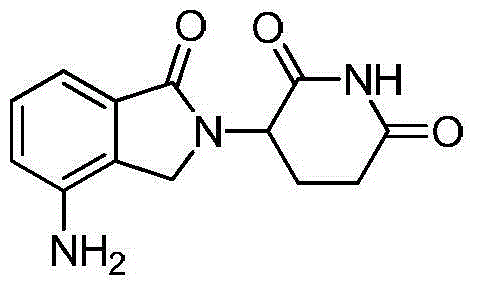
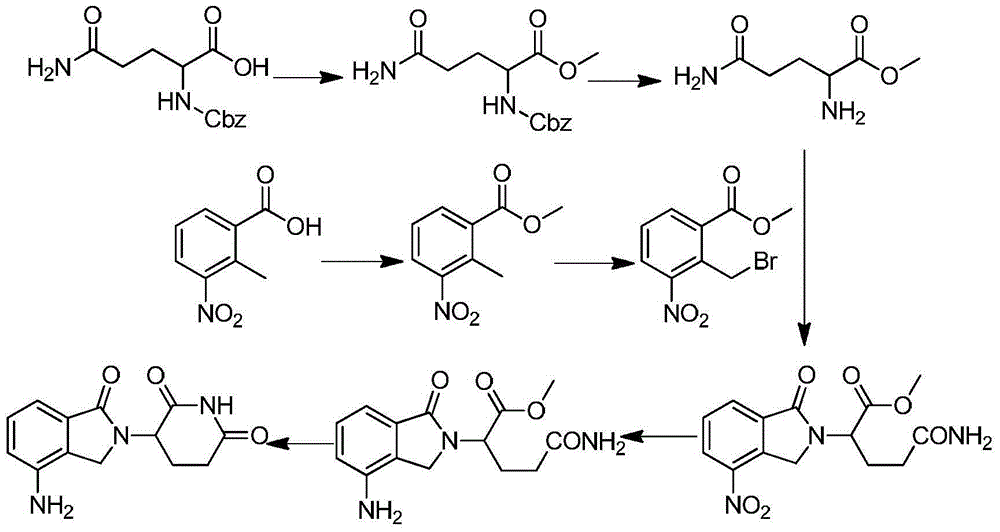
The invention discloses a method for preparing lenalidomide. The method comprises the following steps: firstly, etherifying 2-methyl-3-nitrobenzoic acid to obtain 2-methyl-3-nitrobenzoic acid methyl ester, brominating to obtain 2-brooethyl-3-nitrobenzoic acid methyl ester, reacting L-glutamine and tert-butyl dicarbonate to obtain N-Boc glutamic acid, acquiring 3-amino-2,6-piperidine diketone protected by Boc from N-Boc-glutamic acid in the presence of a condensing agent and a catalyst, further reacting with acid to prepare 3-amino-2,6-piperidine diketone hydrochloride, reacting 3-amino-2,6-piperidine diketone with 2-brooethyl-3-nitrobenzoic acid methyl ester so as to obtain 3-(4-nitryl-1,3 dihydro-1-oxo-2 hydrogen-isobenzazole-2-yl) piperidine-2,6-diketone, and finally reducing, thereby obtaining lenalidomide. The method disclosed by the invention is high in product yield.
Owner:SHANGHAI INST OF TECH
Synthesis of Bcl-2 inhibitor ABT-199
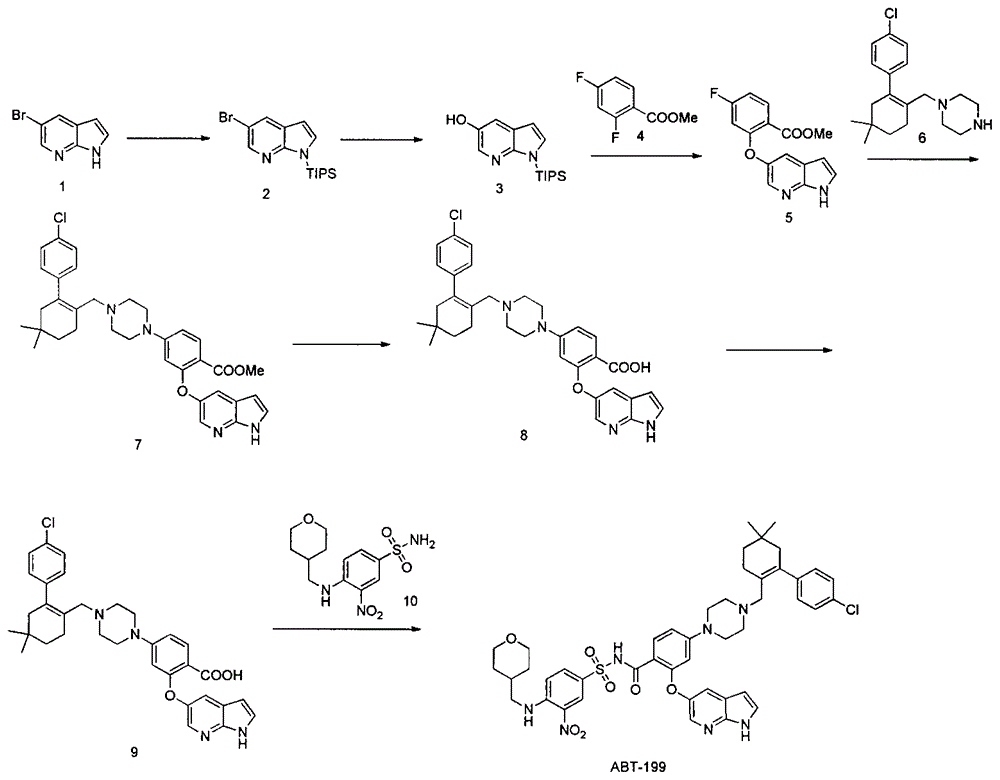
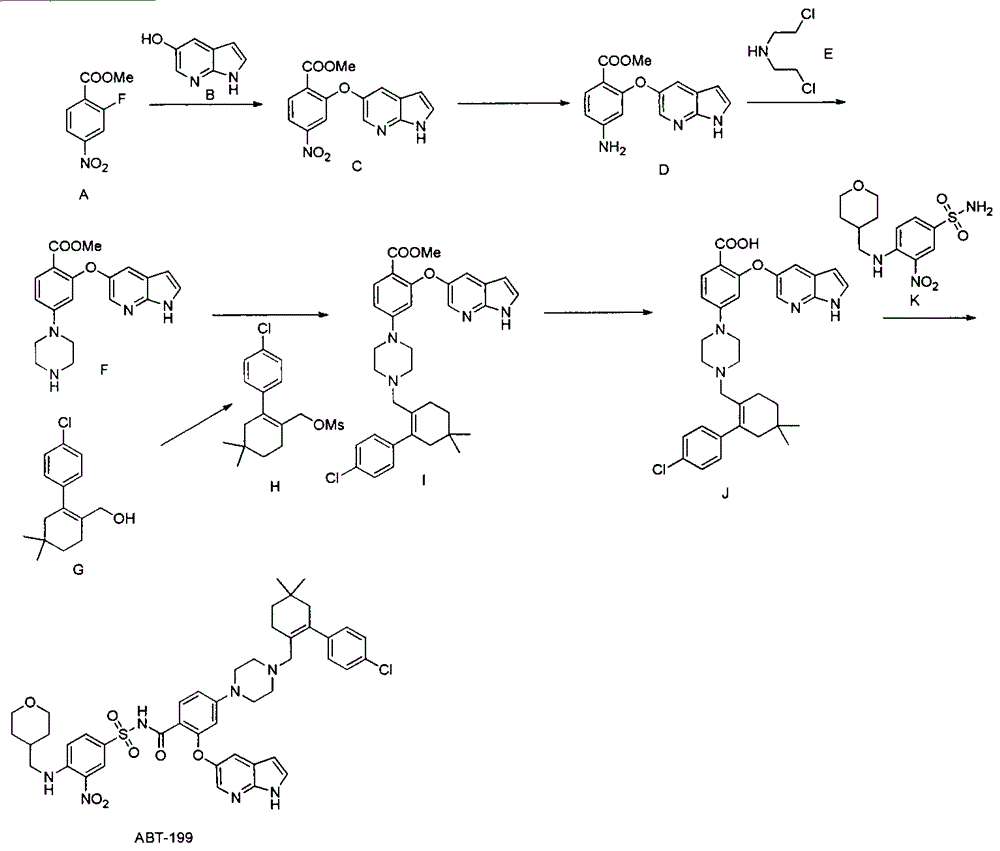
The invention discloses a synthesis method of a Bcl-2 inhibitor ABT-199. The method comprises the following steps: by using methyl 2-fluoro-4-nitrobenzoate and 5-hydroxy-7-azaindole as raw materials, carrying out substitution, reduction, cyclization, substitution, hydrolysis, condensation and the like to synthesize the ABT-199. The method has the characteristics of mild reaction conditions, simple operating technique, low cost and high yield.
Owner:南京正济医药销售有限公司
Method for preparing candestartan
ActiveCN1800179ARaw materials are easy to getSimple operation processOrganic chemistryTert-Butyloxycarbonyl protecting groupOxygen
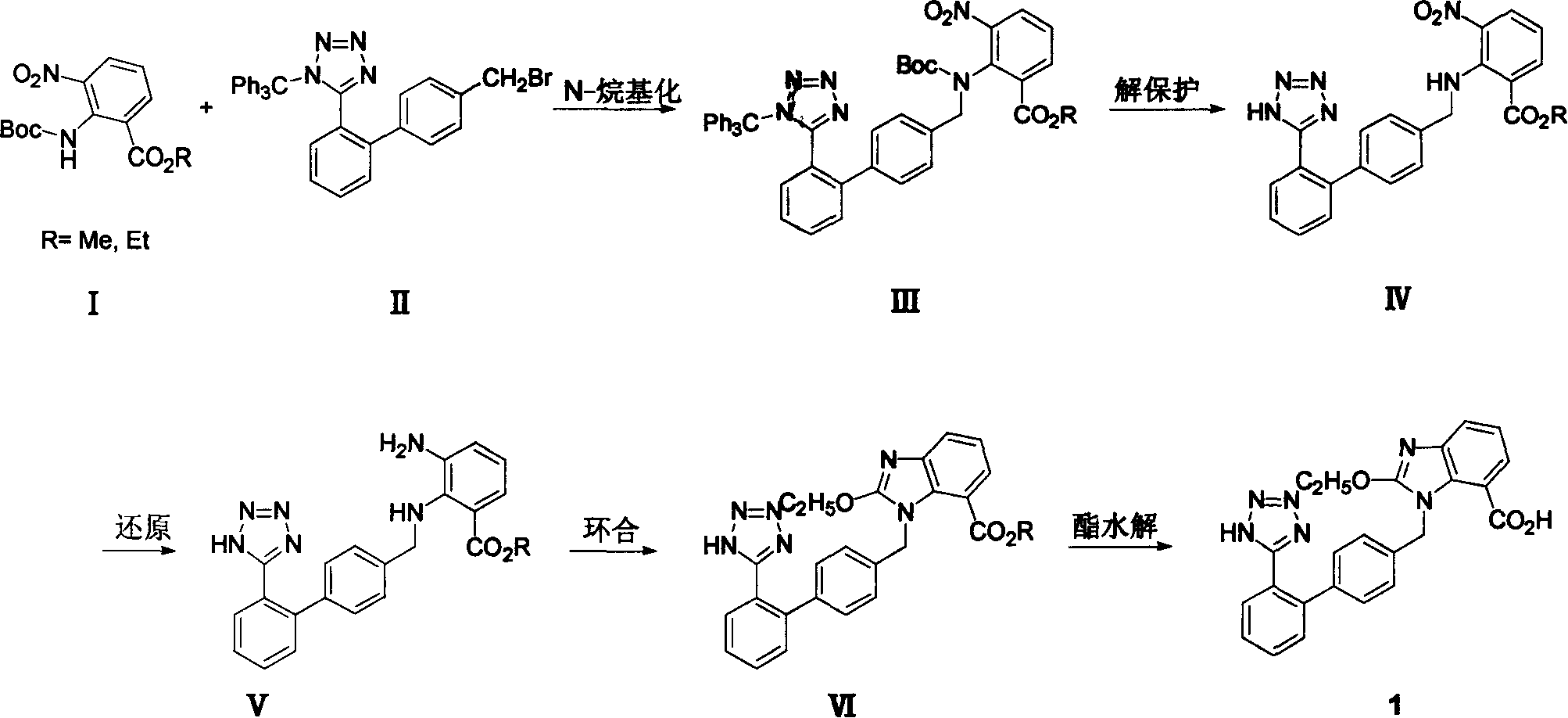
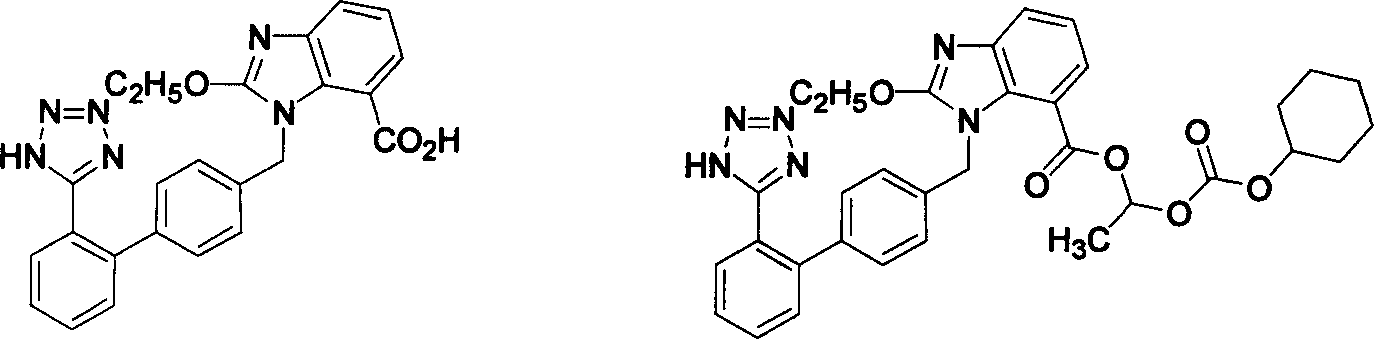
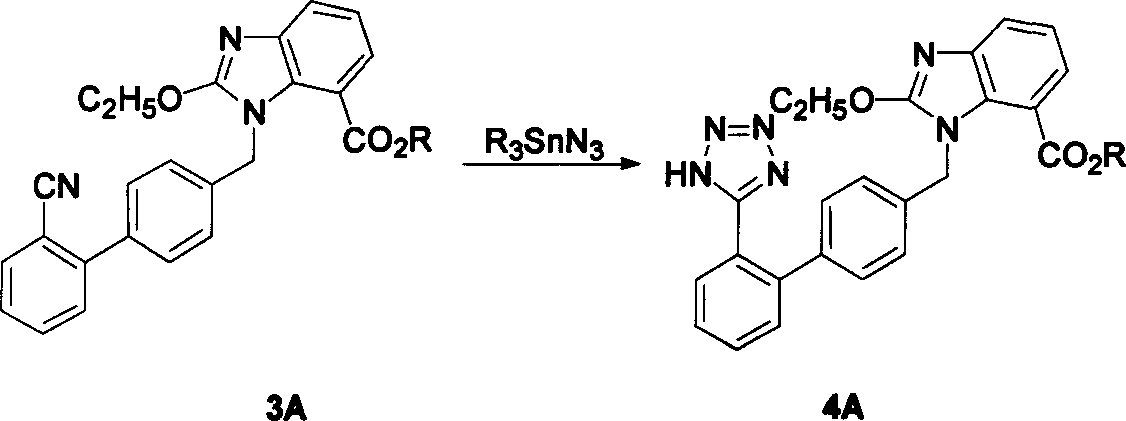
The invention relates to a method for synthesizing ridge sha tan, which uses the 2-tert-butyl ketonic oxygen amido-3-nitro benzoate (I) and N-(trityl )-5-(4'-morphine methylbiphenyll-2-group) tetrazolium (II) as raw material to do N-alkanisation reaction, protecting-released reaction, reduction reaction, ring-closed reaction and ester hydrolytic reaction. <0The protecting-released reaction can slough trityl and tert-butyl ketonic oxygen protect group in the lower aliphatic alcohol organic mixing solution.
Owner:LINHAI TIANYU PHARMA
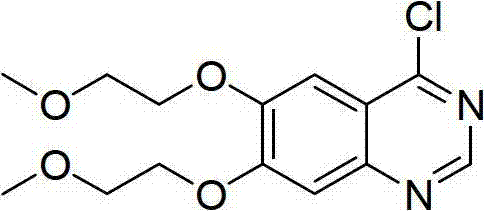
Preparation method for 4-chloro-6,7-bis(2-methoxyethoxy)quinazoline
The invention discloses a preparation method for 4-chloro-6,7-bis(2-methoxyethoxy)quinazoline. The process flow of the method is as follows: A, 3,4-bis(2-methoxyethoxy)benzaldehyde undergoes nitration to produce 4,5-bis(2-methoxyethoxy)-2-nitrobenzaldehyde; B, oxidation is carried out so as to obtain 4,5-bis(2-methoxyethoxy)-2-nitrobenzoic acid; C, esterification is carried out so as to obtain 4,5-bis(2-methoxyethoxy)-2-nitrobenzoate; D, reduction is carried out so as to obtain 4,5-bis(2-methoxyethoxy)-2-aminobenzoate; E, cyclization is carried out so as to obtain 6,7-bis(2-methoxyethoxy)-4(3H)-quinazolinone; and F, chlorination is carried out so as to obtain 4-chloro-6,7-bis(2-methoxyethoxy)quinazoline. The preparation method provided in the invention has the advantages of a concise and practical synthetic route, high yield and a good industrial application prospect.
Owner:ZHEJIANG SCI-TECH UNIV
Device and method for treating organic industrial wastewater based on cavitation technique
PendingCN106145481AImprove digestion rateHigh removal rateWater/sewage treatment by centrifugal separationWater/sewage treatment by irradiationSuspended particlesCavitation
The invention relates to a device and a method for treating organic industrial wastewater based on a cavitation technique, and particularly relates to a device for treating industrial wastewater containing nitrobenzoates and aminobenzenes generated in the production process of toluene diisocynate (TDI). The device comprises a buffer tank, a rotary pulse cavitation device, a membrane filter, a hydraulic cavitation device, an ultraviolet lamp, an ozone generator, a carbon granule filter and a cyclone separator, wherein the rotary pulse cavitation device is used for removing nitrogen containing aromatic compounds from the wastewater through liquid-liquid extraction by using methylbenzene, the membrane filter is used for separating the methylbenzene from the treated wastewater, the ultraviolet lamp and the ozone generator are used for comprehensively purifying the wastewater so as to remove organic impurities, the carbon granule filter is used for removing residual ozone and organic impurities from the wastewater, and the cyclone separator is used for removing suspended particles from the wastewater.
Owner:格斯瑞科技有限公司
Preparation method of lenalidomide
The invention relates to the field of drug synthesis, and particularly relates to a preparation method of a lenalidomide intermediate and lenalidomide. The compound is a drug for treating multiple myeloma. The method comprises the steps of adopting 2-bromomethyl-3-nitrobenzoate and 3-amino-2,6-piperidione hydrochloride as reaction substrates and an inorganic base as an acid-binding agent and obtaining a white to almost white key intermediate 3-(4-nitro-1-oxo-1,3-dihydro-2H-isoindole-2-yl) piperidine-2,6-diketone of the lenalidomide through simple post-treatment; and adopting a mixed solvent of an organic solvent and water as a reaction solvent and carrying out catalytic hydrogenation in the presence of palladium on carbon to prepare the lenalidomide (II). The process route is low in production cost, and a product is high in purity and friendly to environment, and has relatively great implement value and social and economical benefits.
Owner:CHANGZHOU PHARMA FACTORY
Green preparation process of benzocaine
PendingCN108314627AReduce usageHigh purityOrganic compound preparationAmino-carboxyl compound preparationOrganic solventP-nitrobenzoic acid
The invention discloses a green preparation process of benzocaine. The process comprises the steps that p-nitrobenzoic acid and ethanol are subjected to esterification under the catalysis of concentrated sulfuric acid to obtain ethyl p-nitrobenzoate; ethyl p-nitrobenzoate is subjected to catalytic hydrogenation to obtain the benzocaine, wherein catalytic hydrogenation reaction is performed in water; the catalytic hydrogenation reaction temperature is 90 to 110 DEG C; the catalytic hydrogenation reaction pressure is 0.2 to 1.0MPa; the use quantity of water is 3 to 7 times of the weight of ethylp-nitrobenzoate; the weight ratio of ethyl p-nitrobenzoate to a catalyst is (1:0.001)-(1:0.1); the catalyst is a Pd / C catalyst with the mass fraction being 3 to 10 weight percent. Through the catalytic hydrogenation reaction, the use of organic solvents is avoided, so that the whole benzocaine preparation becomes a green process; the proper use quantity of water is selected, so that high reactionyield and product purity can be obtained.
Owner:CHANGZHOU YONGHE FINE CHEM
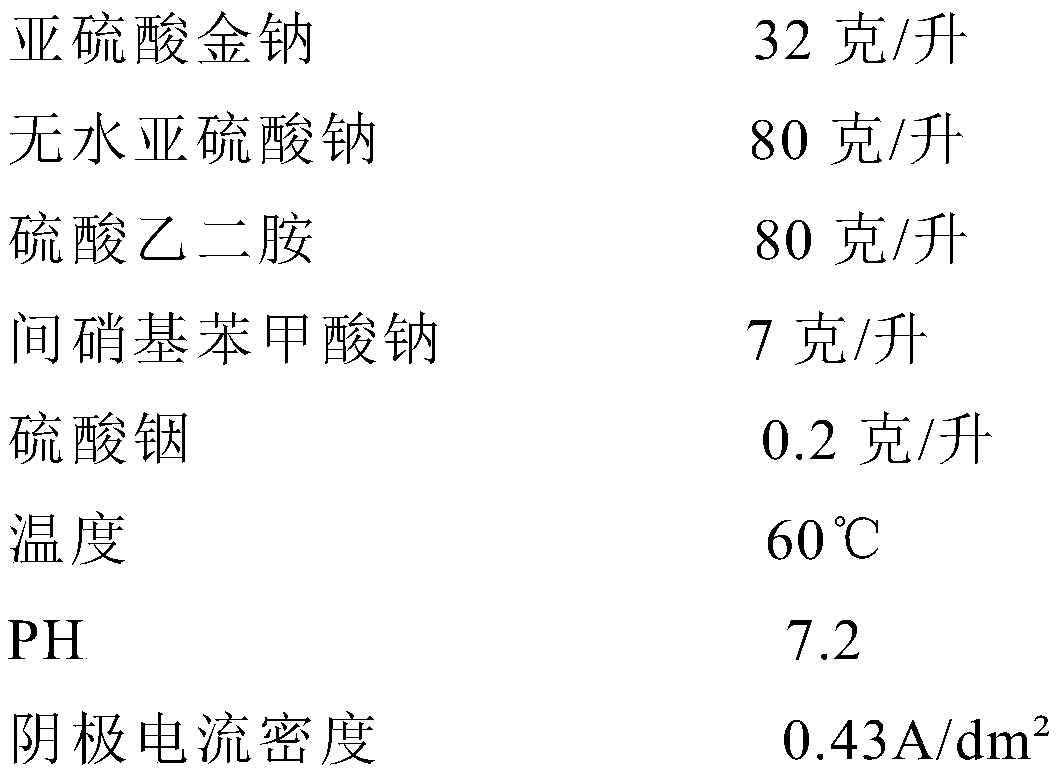
Electroforming liquid for gold cyanide-free electroforming process
The invention relates to electroforming liquid for a gold cyanide-free electroforming process. The electroforming liquid is prepared from the components: 17-35 g / l of sodium gold sulfide, 50-80 g / l ofethylenediamine sulfate, 50-90 g / l of anhydrous sodium sulfite, 3-10 g / l of sodium 3-nitrobenzoate and 0.1-1 g / l of indium sulfate, the electroforming liquid for the gold cyanide-free electroformingprocess is prepared, and then gold products are formed through electroforming. According to the electroforming liquid for the gold cyanide-free electroforming process, the problems of difficult operation, unstable electroforming liquid, short service life, and the like of current cyanide-free gold electroforming processes are solved, thus the cyanide-free hard gold electroforming process with stable electroforming liquid, easy operation, and wide application to practical production is achieved, and the difficulty and production cost of cyanide-free hard gold electroforming production are lowered.
Owner:深圳市昊扬电铸技术开发有限公司
Process for synthesizing carbapenem using raney nickel
Convenient method for obtaining carbapenem by hydrogenation with Raney Nickel, as an alternative to the known catalytic hydrogenation conducted under hydrogen overpressure in the presence of Palladium, starting from corresponding protected intermediates such as p-nitrobenzylesters and with optional suitable protections of any primary and secondary amino functions structurally present.
Owner:ACS DOBFAR SPA
Finishing agent having function of reinforcing air permeability of textiles and preparation method of finishing agent
InactiveCN106637950AStrong oil repellencyImprove breathabilityLiquid repellent fibresUltrasonic dispersionMethyl oleate
The invention discloses a finishing agent having a function of reinforcing the air permeability of textiles and a preparation method of the finishing agent. The finishing agent adopts methyl oleate, isophthalic acid diphenyl ester, dimethiconol stearate and aqueous polyurethane as main components. According to the finishing agent disclosed by the invention, a naphthalene sulfonate-formaldehyde condensation compound, aluminum tartrate, phthalic acid ester, polyglycerol monostearate, 2-fluorine-5-nitrobenzoate, sodium persulfate, sodium laureth ulfate, amino silicone oil, 6-hydroxyflavone, nanometer jade particles, a surfactant, a thickening agent and deionized water are added, and technologies including heating, stirring, ultrasonic dispersion, ball milling, reaction under nitrogen environment and the like are used for assisting, so that after the finishing agent prepared by the preparation method disclosed by the invention is used, the textiles have favorable air permeability, the evaporating speed of water is high, the oil resistance is high, requirements of the industry can be met, and the textiles have good application prospects.
Owner:WUJIANG BEISHE SHENGYUAN TEXTILE PROD AUXILIARIES PLANT
Preparation method of lenalidomide for treating multiple myeloma
ActiveCN107337666AQuick and easy to manufactureSimple and fast manufacturing methodOrganic chemistryAntineoplastic agentsZinc bromideOrganic solvent
The invention relates to a drug synthesis technology and discloses a preparation method of lenalidomide for treating multiple myeloma. The preparation method comprises the following steps: firstly, carrying out hybrid reaction on 2-methyl-3-methyl 3-nitrobenzoate and 3-amino-2,6-dioxopiperidine in an organic solvent in the presence of tetramethylethylenediamine and zinc bromide, so as to obtain 3-(7-nitro-3-oxo-1H-isoindol-1-yl) piperidine-2,6-dione; secondly, carrying out catalytic hydrogenation reduction on 3-(7-nitro-3-oxo-1H-isoindol-1-yl) piperidine-2,6-dione, so as to obtain the lenalidomide. The preparation method of the lenalidomide, disclosed by the invention, has the advantages of mild conditions, simple reaction steps and environment friendliness, so that the preparation method is more suitable for industrial production.
Owner:SHANGHAI WANXIANG PHARMA
Preparation method of imidocarb
The invention relates to a preparation method of imidocarb. The preparation method comprises the steps of dissolving methyl-m-nitrobenzoate into a solvent, enabling methyl-m-nitrobenzoate and ethylenediamine to be subjected to cyclization reaction at the temperature of 55-65 DEG C for 3-6h under the action of a catalyst, and separating to prepare m-nitroimidazoline; dissolving m-nitroimidazoline into a water solution of methanol, adding zinc powder under an acidic condition, after the addition is ended, carrying out reduction reaction for 1-2h while stirring at normal temperature, and separating and purifying to prepare m-aminoimidazoline; dissolving m-aminoimidazoline into a water solution of tetrahydrofuran, regulating the pH value to 7-10, adding triphosgene, reacting at the temperature of 10-40 DEG C for 2-6h, filtering and drying to obtain imidocarb. Methyl-m-nitrobenzoate is used as a starting raw material, and zinc powder is used as a reducing agent, so that the preparation method is mild in reaction condition, convenient in operation and relatively low in cost.
Owner:山东久隆精细化工有限公司 +2
Simple and convenient production method of rucaparib
ActiveCN110229162AMild process conditionsEnsure safe productionOrganic chemistrySulfonyl chlorideFormate
The invention relates to a simple and convenient production method of rucaparib. The method comprises the steps of conducting condensation on 5-fluoro-2-methyl-3-nitrobenzoate and 4-(N-methyl-N-PG-aminomethyl)benzoate under the action of an alkaline, subjecting an obtained condensation product and ethylene oxide to a hydroxyethylation reaction, carrying out sulfonyl chloride protection and catalytic hydrogenation indole cyclization to produce 2-(4-methylaminomethyl)phenyl-3-(2-sulphonate)ethyl-6-fluoro-1H-indole-4-formate, and then subjecting the 2-(4-methylaminomethyl)phenyl-3-(2-sulphonate)ethyl-6-fluoro-1H-indole-4-formate and ammonia to an SN2 substitution reaction and an amidation reaction to produce the rucaparib through a one-pot method. The raw materials of the method are cheap andeasy to obtain, and the method is short in technology process, simple and convenient to operate, little in wastewater quantity and environmentally friendly, and benefits industrial production of rucaparib.
Owner:XINFA PHARMA
Synthesis method of benzocaine
InactiveCN106699579AHigh yieldImprove teaching effectOrganic compound preparationAmino-carboxyl compound preparationP-nitrobenzoic acidSynthesis methods
Relating to the field of chemical engineering, the invention in particular relates to a synthesis method of benzocaine. The method includes the steps of: synthesis of p-nitrobenzoic acid; synthesis of ethyl 4-nitrobenzoate; and synthesis of benzocaine. Through improvement of benzocaine synthetic experiment method, the invention specifically solves the problems of low yield, tedious reaction aftertreatment and the like in conventional preparation experiments, and the improved experimental operation method not only can achieve high yield preparation of benzocaine, enhance experimental efficiency, but also effectively improves the teaching effects of the experimental course, and is suitable for chemical experimental course teaching.
Owner:SHAANXI ALLIANCE LOGISTICS
Fluorescent probe for detecting hydrogen persulfide, synthetic method of fluorescent probe and application of fluorescent probe
ActiveCN109734711AImprove stabilityExcellent optical propertiesOrganic chemistryFluorescence/phosphorescenceSynthesis methodsFluorescence
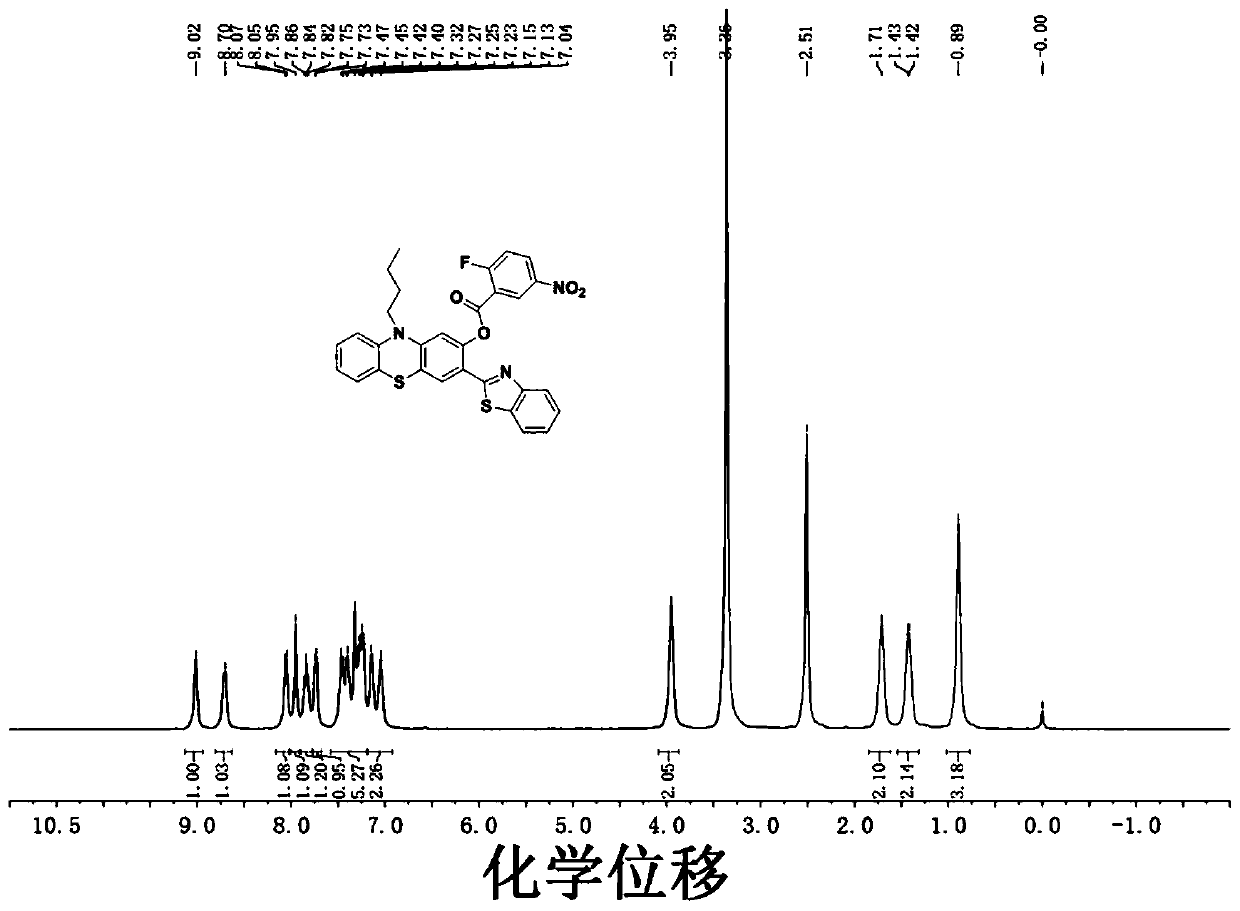
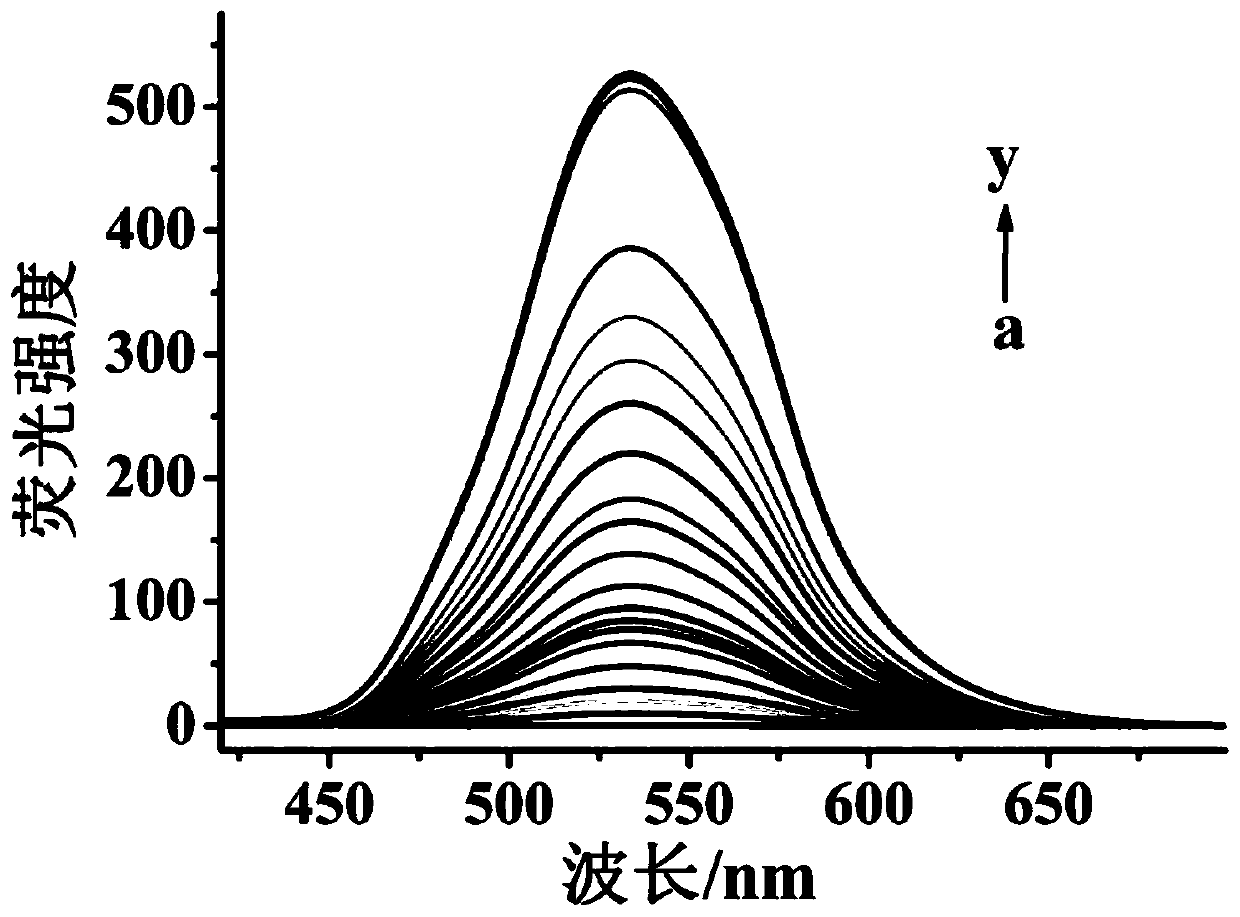
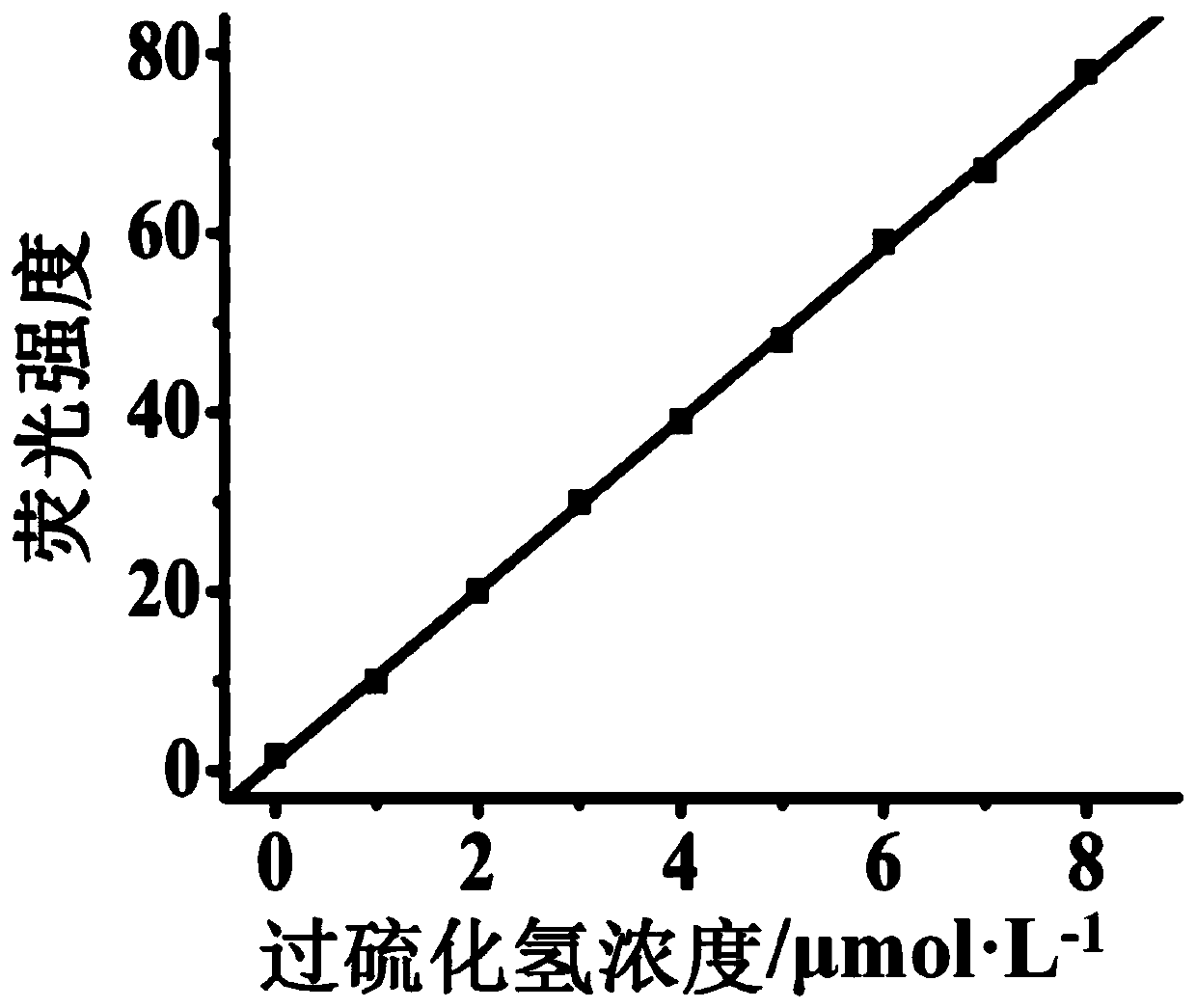
The invention discloses a fluorescent probe for detecting hydrogen persulfide, a synthetic method of the fluorescent probe and an application of the fluorescent probe, and belongs to the technical field of chemical analysis and detection. A benzothiazole phenothiazine fluorogen with green fluorescence emission reacts with 2-fluoride-5-nitrobenzoic acid to obtain the probe which has a general structural formula as shown in the specification. The fluorogen of the probe is benzothiazole phenothiazine, and a hydrogen persulfide response group is 2-fluoride-5-nitrobenzoate. Probe molecules have high selectivity and sensitivity to the hydrogen persulfide, detection range is 0-8 micromoles*L<-1>, and detection limit is 26 nanomoles*L<-1>. The probe can be used for detecting the hydrogen persulfide in water, soil and cells.
Owner:SHANGQIU NORMAL UNIVERSITY
Spectrophotometric assay for human histone deacetylase 8
InactiveUS20080286815A1Microbiological testing/measurementBiological material analysisEllman's reagent2-nitro-5-thiobenzoate
The present invention relates to the development of a novel assay for determining the activity of human histone deactylase 8 (HDAC8). As shown in FIG. 3, thioactyl-lysine-containing peptide is used as the substrate for the HDAC8-catalyzed dethioacetylation reaction. Thioacetate, which is formed during this reaction, is subsequently reacted with Ellman's reagent, 5,5′-dithiobis(2-nitrobenzoate), (DTNB) with a quantitative formation of 2-nitro-5-thiobenzoate (TNB). The concentration of thioacetate formed during the conversion reaction can be quantified by measuring the absorbance of TNB at 412 nm.
Owner:THE UNIVERSITY OF AKRON
Method for preparing tolvaptan intermediate
ActiveCN102690211ASimple processing methodQuality improvementOrganic compound preparationCarboxylic acid amides preparationHydrolysisMethyl group
The invention relates to a method for preparing a tolvaptan intermediate. The method comprises the following steps of: reacting 2-methyl-4-nitrobenzoic acid used as an initial raw material and alcohols under the action of a catalyst to generate 2-methyl-4-nitrobenzoate, and reacting under the action of palladium-carbon catalyst by using ammonium formate as hydrogen donor to obtain 2-methyl-4-aminobenzoate compounds; and reacting the 2-methyl-4-aminobenzoate compounds and o-toluoyl chloride under the alkaline condition to generate 2-methyl-4-N-(2-methylbenzoyl)benzoate, adding alkali and a catalyst at presence of water to perform hydrolysis, extracting a water layer by using an organic reagent for one to two times, after layering, collecting a water-layer solution, adjusting the pH of the water-layer solution to be 5 to 6 to separate solid out, filtering an aqueous solution out to obtain solid remnant, and drying to obtain a target product. By the method, the quality and yield of a product are improved; a method for treating the intermediate is simplified; and a simple and feasible process method is provided for industrial production.
Owner:ZHENGZHOU MINGZE MEDICAL TECH
An environment-friendly production process of p-aminobenzamide
ActiveCN109867604AReactivity NoneCoking phenomenon noOrganic compound preparationCarboxylic acid amides preparationAlcoholHydrogen
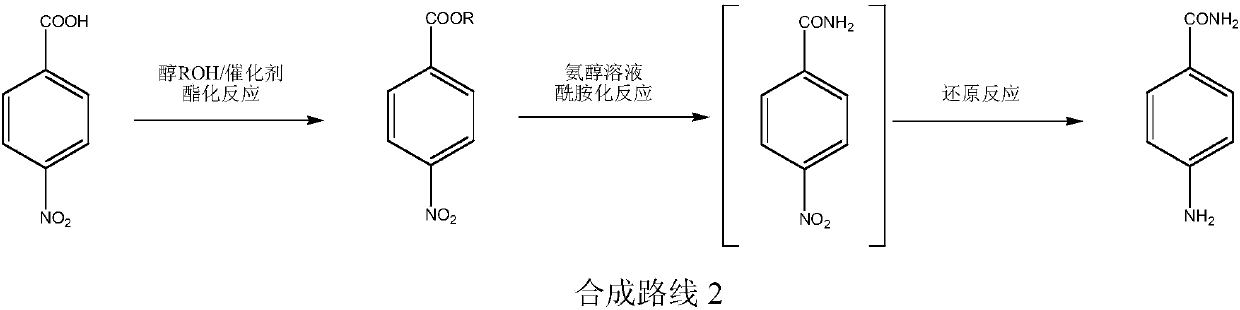
The invention relates to an environment-friendly production process of p-aminobenzamide, which comprises the following steps: carrying out catalytic esterification reaction on p-nitrobenzoic acid andalcohol to generate p-nitrobenzoate, and carrying out amidation reaction on the p-nitrobenzoate in the presence of a hydrogenation catalyst and hydrogen in an ammonia-alcohol solution to prepare the p-aminobenzamide. The process has the advantages of cheap and easily available raw materials, low wastewater amount, high operation safety, high reaction selectivity, high product yield, high product purity and low cost, and is simple to operate.
Owner:XINFA PHARMA
Sulfur-free propellant compositions
ActiveUS7344610B2Eliminate the problemEasy to cleanNitrated aromatic explosive compositionsInorganic oxygen-halogen salt explosive compositionsBurn rateGluconic acid
Sulfur free propellant compositions for use as a blackpowder substitute in firearms, munitions, and pyrotechnics to form combustion byproducts that are water soluble and are free of corrosive sulfur compounds. Formulations include an ignition aid of a gluconic acid salt or an alkali metal nitrobenzoate salt or a mixture of the two salts in combination with various known oxidizing and reducing agents and with various known propellant additives. The oxidizing and reducing agents may be selected from the group consisting of carbon, lactose, potassium nitrate, potassium perchlorate, sodium benzoate, and mixtures thereof. The propellant additives may be selected from the group consisting of binders, burning rate modifiers, flow agents, colorants, coating agents, moisture retardants and mixtures thereof.
Owner:HODGDON POWDER COMPANY
A method of synthesizing a neratinib intermediate, 3-cyano-4-chloro-6-amino-7-ethoxyquinoline
A method of synthesizing a neratinib intermediate that is 3-cyano-4-chloro-6-amino-7-ethoxyquinoline is disclosed. The method includes (1) subjecting methyl 4-ethoxy-2-chloro-5-nitrobenzoate and 3-amino acrylonitrile to a condensation reaction under the action of a catalyst 1 to obtain 2-(4-ethoxy-2-chloro-5-nitrobenzoyl)-3-amino acrylonitrile; (2) subjecting the 2-(4-ethoxy-2-chloro-5-nitrobenzoyl)-3-amino acrylonitrile to a cyclization reaction to obtain 3-cyano-4-oxo-6-nitro-7-ethoxy-1,4-dihydroquinoline; (3) subjecting the 3-cyano-4-oxo-6-nitro-7-ethoxy-1,4-dihydroquinoline and phosphorus oxychloride to a chlorination reaction to obtain 3-cyano-4-chloro-6-nitro-7-ethoxyquinoline; and (4) subjecting the 3-cyano-4-chloro-6-nitro-7-ethoxyquinoline and hydrazine hydrate to a reduction reaction under the action of a catalyst 2 to obtain a target product. According to the method, synthetic steps are few, reaction conditions are mild, agents are cheap and easily available, operation is simple and the total yield is high. The method provides a novel route for preparation of neratinib and the intermediate.
Owner:山东金吉利新材料有限公司
Method for preparing 5-methyl carboxylate-6-benzoltriazolylmethoxy
InactiveCN102399198ARaw materials are easy to getEasy to operateOrganic chemistryAcetic anhydrideMethylene Dichloride
The invention discloses a method for preparing 5-methyl carboxylate-6-benzoltriazolylmethoxy. The method comprises the following steps of taking 2-methoxyl-4-aminobenzoate as a raw material, adding acetic anhydride and glacial acetic acid with a dissolved quantity and concentrated nitric acid with a nitration quantity in the raw material, performing a nitration reaction to prepare an intermediate of 2-methoxyl-4-amino-5-nitrobenzoate; taking 2-methoxyl-4-amino-5-nitrobenzoate as a raw material, adding methanol with a dissolved quantity and Raney nickel with a catalytic amount, controlling the pressure at 0.5-0.8 Mpa, putting hydrogen into the mixture, performing a hydrogenation reaction, pouring methanol aqueous solution containing sulfuric acid into the mixture, adding ices to separate out crystals, filtering and drying the crystal to obtain the intermediate 2-methoxyl-4,5-diaminobenzoate; adding the 2-methoxyl-4,5-diaminobenzoate in concentrated hydrochloric acid, adding sodium nitrite solution in the mixture drop by drop at a temperature of 0-5 DEG C, after the adding of the sodium nitrite solution is finished, heating the mixture to 30-35 DEG C and agitating the mixture for 1-2 hours; cooling and filtering the mixture, obtaining solid; washing the solid with methylene dichloride and water in sequence, obtaining the 5-methyl carboxylate-6-benzoltriazolylmethoxy. According to the method provided by the invention, the raw materials are easy to get, the operation is simple, the cost is low, the industrialization production is easy to realize, the product yield is high and more than 80%, therefore, the method has a good practicability and can produce good economic benefit and social benefit.
Owner:苏州诚和医药化学有限公司
Preparation method of nizofenone
InactiveCN102718716AOvercome the disadvantage of large steric hindrance in the Friedel-Crafts reactionMild reaction conditionsOrganic chemistryNizofenoneReaction step
The invention provides a preparation method of nizofenone. The nizofenone is prepared by taking 2-bromo (or chloro)-5-nitrobenzoate and 2-(N, N-diethyl)methylimidazole as starting materials, substituting, hydrolyzing, acylating and performing Friedel reaction. In the first step of the preparation method, a benzene ring and imidazole ring-containing compound is prepared, so that the subsequent reaction steps are carried out under mild conditions, and the yield is greatly improved, so as to reduce the production cost.
Owner:湖南尔文水电建材有限公司
Process for production of quinuclidine compounds
InactiveUS20130060036A1High yieldLow environmental burdenDigestive systemImmunological disordersBenzoic acidP-nitrobenzoic acid
Provided is a production method for a cis-QMF, which has a low environmental burden and is industrially advantageous. Specifically provided is a production method for a cis-type 2-alkylspiro(1,3-oxathiolane-5,3′)quinuclidine hydrochloride, including: reacting a cis-trans isomer mixture of 2-alkylspiro(1,3-oxathiolane-5,3′)quinuclidine with p-nitrobenzoic acid; resolving the resultant product to produce a cis-type 2-alkylspiro(1,3-oxathiolane-5,3′)quinuclidine p-nitrobenzoate; and converting the p-nitrobenzoate into a hydrochloride.
Owner:DAIICHI SANKYO CO LTD
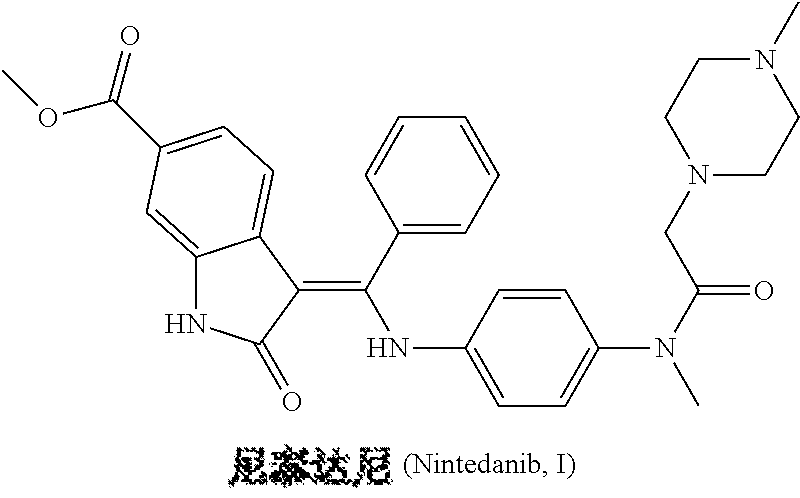
Preparation method of nintedanib
Disclosed is a preparation method of nintedanib (I), comprising the following steps: carrying out a condensation reaction on 4-(R acetate-2-yl)-3-nitrobenzoate (II) and trimethyl orthobenzoate to obtain (E)-4-[(2-methoxybenzylidene) R acetate-2-yl]-3-nitrobenzoate (III); carrying out a substitution reaction on the compound (EI) and N-(4-aminophenyl)-N-methyl-2-(4-methyl piperazine-1-yl) acetamide (IV) under the action of an acid-binding agent to generate (Z)-4-{[2-(N-methyl-2-(4-methyl piperazine-1-yl) acetamido-aniline) benzylidene] R acetate-2-yl}-3-nitrobenzoate (V); and sequentially carrying out reduction reactions and cyc-lization reactions on the compound (V) to prepare the nintedanib (I). The preparation method has an easily obtained raw material and a simple process, is economical and environmentally friendly, and is suitable for industrial production.
Owner:SUZHOU MIRACPHARMA TECH
Catalyst for synthesizing p-dimethylaminobenzoate and preparation method and application thereof
ActiveCN109453784AIncreased nickel contentLow costOrganic compound preparationAmino-carboxyl compound preparationNitro compoundHydrogenation reaction
The invention discloses a catalyst for synthesizing p-dimethylaminobenzoate and a preparation method and application thereof. The catalyst contains metal of Ni and Pd and a carrier of aluminum oxide.In the catalyst, mass percent content of the Ni is 0.8-1.1%, mass percent content of the Pd is 0.2-0.5%, and the rest is the aluminum oxide. The invention further discloses the preparation method andthe application of the catalyst. In the presence of the prepared catalyst, p-nitrobenzoate and formaldehyde as raw materials are subjected to the nitro hydrogenation and methyl addition reaction by one step to generate a target product, wherein a molar yield is high. In a preparation process, subsection filling and subsection feeding modes are used. At the upper end of a reaction tube, the catalyst mainly performs a nitro hydrogenation reaction which is an exothermic reaction, and the reaction is violent. A material with reaction heat after the previous reaction enters the next catalyst. The material is mainly an amino compound, and a methyl addition reaction is performed with theformaldehyde. A small amount of residual unreacted nitro compound is rapidly converted into the amino compoundon the undiluted catalyst.
Owner:XIAN CATALYST NEW MATERIALS CO LTD
Environment-friendly copper-tin alloy coating deplating liquid and method deplating method
The invention relates to an environment-friendly copper-tin alloy coating deplating liquid and a deplating method thereof. Per liter of deplating liquid comprises the following components by mass concentration: 150-250g / L sodium p nitrobenzoate, 10-30g / L ammonium acetate, 10-30ml / L propyl alcohol, 100-150ml / L glycerol, 6-10ml / L ethylene diamine and 1-3 g / L ammonium thiocyanate. The color of a matrix after deplating of the deplating liquid is fundamentally unchanged; the deplating liquid is stable; a coating is deplated completely and efficiently; a technology is simple; the surface of the matrix of a plating piece is not corroded; no harmful gas is generated; the service cycle is long; the cost can be saved; and the deplating liquid and the deplating method conform to the aims of environmental protection and conservation required in the society.
Owner:FOSHAN UNIVERSITY
Preparation method of oxybuprocaine hydrochloride
InactiveCN106810463ALow costOrganic compound preparationAmino-carboxyl compound preparationHydrazine compoundHigh pressure
The invention discloses a preparation method of oxybuprocaine hydrochloride. The preparation method includes following steps: in the presence of alkali I, subjecting 3-hydroxy-4-ethyl nitrobenzoate and bromobutane to electrophilic substitution reaction to obtain 3-butoxy-4-ethyl nitrobenzoate; under alkaline condition, enabling 3-butoxy-4-ethyl nitrobenzoate to be in hydrolysis reaction, and adjusting pH value of a system after hydrolysis reaction to 1-3 to obtain 3-butoxy-4-nitrobenzoic acid; in the presence of alkali II, subjecting the 3-butoxy-4-nitrobenzoic acid and diethylamino ethyl chloride to electrophilic substitution reaction to obtain 4-nitro-2-butoxy nitrobenzoic acid-2-(diethylamino) ethyl ester, and subjecting the 4-nitro-2-butoxy nitrobenzoic acid-2-(diethylamino) ethyl ester to reduction reaction under action of ferric trichloride and hydrazine hydrate to obtain oxybuprocaine hydrochloride. 3-hydroxy-4-ethyl nitrobenzoate is adopted as a raw material to obtain a target product through four steps of conventional reaction; each step of reaction does not need high-pressure condition, and needed raw materials are all conventional compounds, so that the preparation method is easy to obtain and low in cost.
Owner:SHENZHEN OASIS PHARMA
The preparation method of tolvaptan intermediate
ActiveCN102690211BSimple processing methodQuality improvementOrganic compound preparationCarboxylic acid amides preparationMethyl groupHydrolysis
The invention relates to a method for preparing a tolvaptan intermediate. The method comprises the following steps of: reacting 2-methyl-4-nitrobenzoic acid used as an initial raw material and alcohols under the action of a catalyst to generate 2-methyl-4-nitrobenzoate, and reacting under the action of palladium-carbon catalyst by using ammonium formate as hydrogen donor to obtain 2-methyl-4-aminobenzoate compounds; and reacting the 2-methyl-4-aminobenzoate compounds and o-toluoyl chloride under the alkaline condition to generate 2-methyl-4-N-(2-methylbenzoyl)benzoate, adding alkali and a catalyst at presence of water to perform hydrolysis, extracting a water layer by using an organic reagent for one to two times, after layering, collecting a water-layer solution, adjusting the pH of the water-layer solution to be 5 to 6 to separate solid out, filtering an aqueous solution out to obtain solid remnant, and drying to obtain a target product. By the method, the quality and yield of a product are improved; a method for treating the intermediate is simplified; and a simple and feasible process method is provided for industrial production.
Owner:ZHENGZHOU MINGZE MEDICAL TECH
Method of preparing dihalogen nitrobenzoate using dihalogen methyl nitrobenzene oxidation
InactiveCN100999457AReduce manufacturing costOperational securityOrganic compound preparationCarboxylic compound preparationSocial benefitsToluene oxidation
This invention provides a method of using dipl-halogena nitrotoluene to prepare dipl-halogena nitrobenzoic acid. Mix dipl-halogena nitrotoluene with nitric acid under normal temperature, and then take reaction at 110 to 300 deg C and 0 to 10MPa, to gain dipl-halogena nitrobenzoic acid. The mixture ratio (mol / mol) of nitric acid and dipl-halogena nitrotoluene is 1:21 to 100; the nitric acid concentration of 4% to 70%. Since the invention at room temperature and atmospheric pressure directly mix the materials, so operating temperature is low than the start temperature many, thus making operation simple and safe, the low production costs and high-yield, the reaction residue can be reused, little pollution, and good economic and social benefits.
Owner:YANCHENG SHIHONG CHEM
Preparation method of m-phenylenediamine
InactiveCN111018720AAvoid it happening againAvoid Hazardous ProcessesOrganic compound preparationCarboxylic acid amides preparationBenzoic acidNitrobenzene
The invention relates to a preparation method of m-phenylenediamine, which comprises the following steps: by using benzoic acid as an initial raw material, reacting with an alcohol reagent under the action of an acid to esterify, and nitrifying to obtain a m-nitrobenzoate, or by using benzoic acid as an initial raw material, nitrifying, and reacting with an alcohol reagent under the action of an acid to esterify to obtain the m-nitrobenzoate; carrying out aminolysis of the m-nitrobenzoate to obtain 3-nitrobenzamide, reacting with a hypohalite or a halogen in an alkaline solvent to obtain m-phenylenediamine. Compared with the prior art, the method has the advantages that generation of explosive compound dinitrobenzene and generation of a large amount of waste acid are avoided, the whole production process is stable, and the yield is high.
Owner:SHANGHAI JIAO TONG UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com