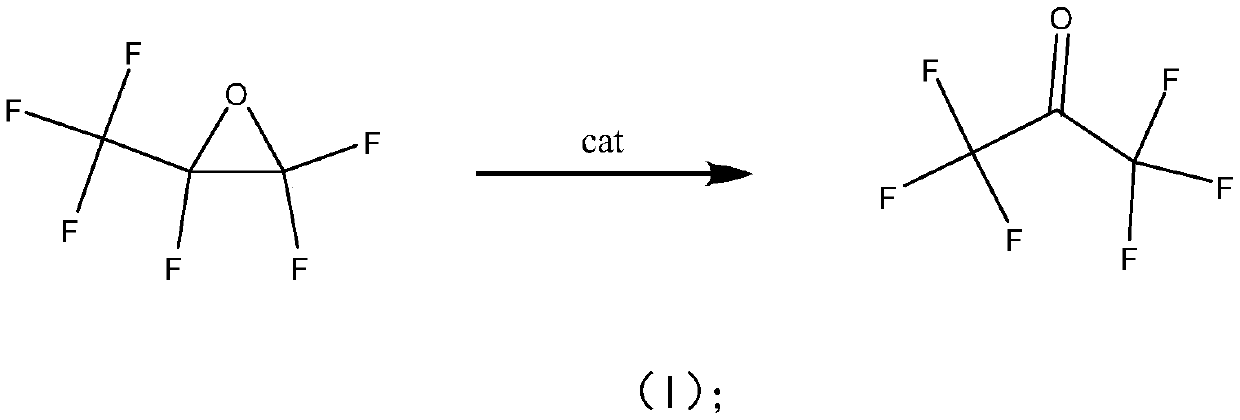
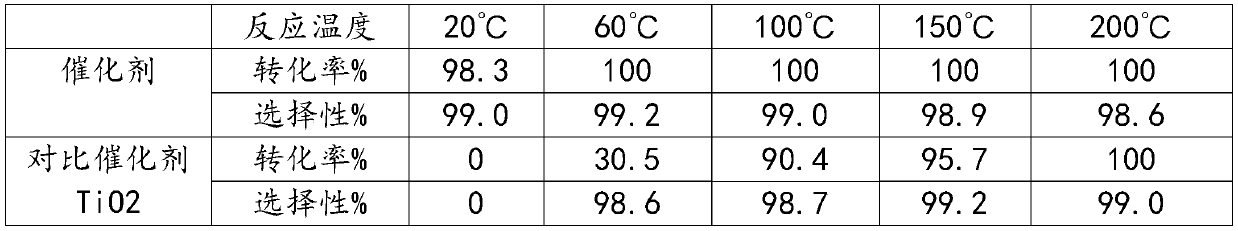
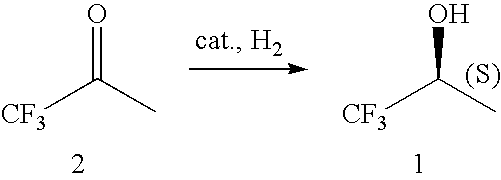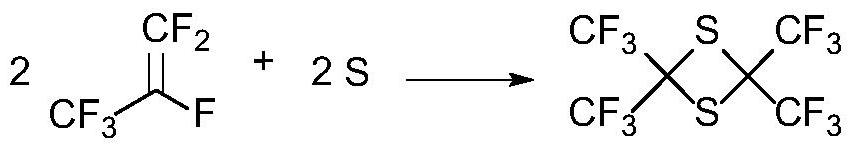Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
58 results about "Fluoroacetone" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Fluoroacetone is an organofluorine compound with the chemical formula C₃H₅FO. In contrast to trifluoroacetone, the compound has one fluorine atom. Under normal conditions, the substance is a colorless to light yellow liquid. Fluoroacetone is also a highly toxic and flammable compound. Fumes of fluoroacetone can form an explosive mixture with air.
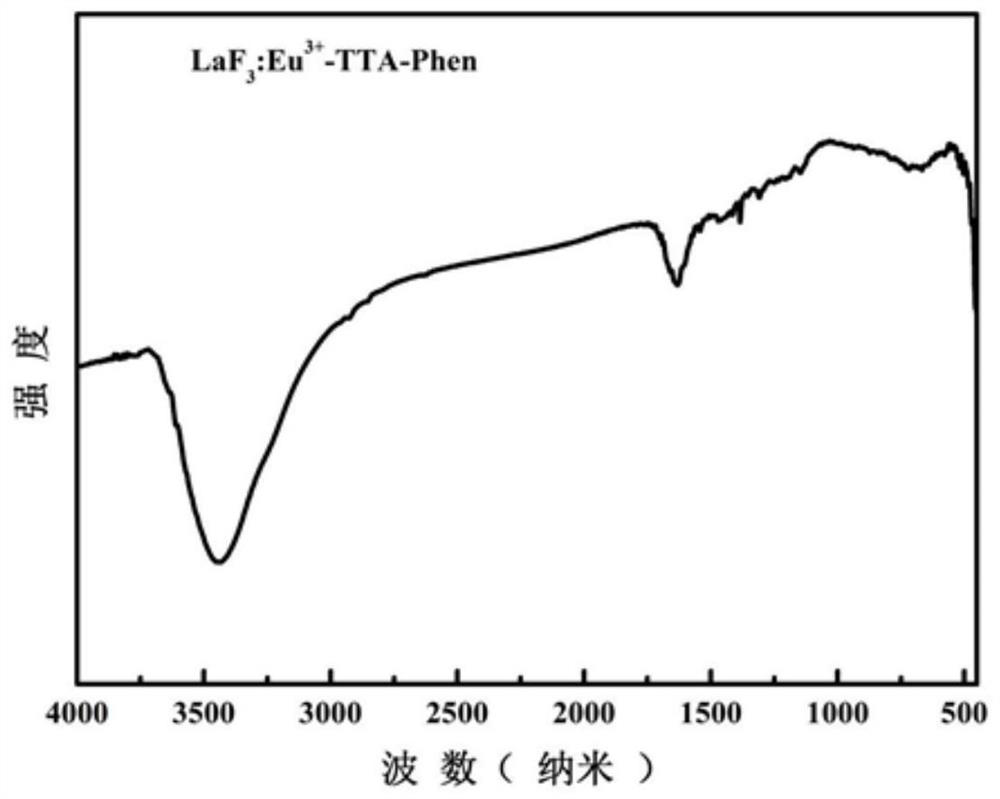
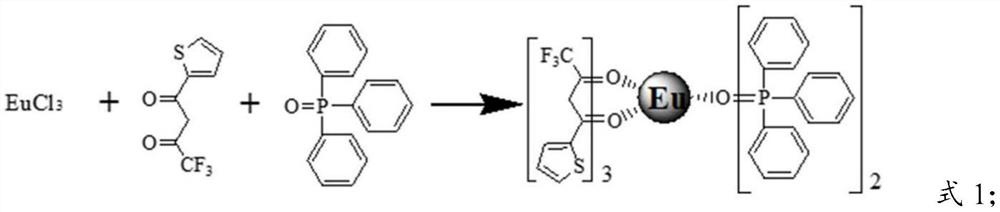
Rare-earth organic complex light conversion agent and method of preparing the same
ActiveCN101358128ALow priceGood spectral matchingGroup 3/13 element organic compoundsLuminescent compositionsFiltrationUltraviolet lights
The present invention discloses a bionical rare earth organic complex light conversion agent and a preparation method thereof, and the composition of the bionical rare earth organic complex light conversion agent is as follows: SmM<1-x>(TTA)L. The light conversion agent chooses the easily obtained, cheap samarium ion as a central ion, lanthanum La<3 plus>, gadolinium Gd<3 plus> and yttrium Y<3 plus> as heterocaryotic central ions, organic compound (Alpha-thenoyltrifluoroaceton) with the lowest excited triplet level matching the Sm<3 plus> excited level as a ligand and o-phenanthroline, 2,2'-bipyridyl, trioctylphosphine oxide, triphenylphosphine oxide, etc. as second ligands. The method for preparing the light conversion agent includes the following steps: dissolution, stirring and reaction, deposition, filtration, washing, drying, grinding and finished product, and the appearance of the product is white or light yellow. The light conversion agent provided by the present invention can convert ultraviolet light into red light with a wavelength between 620nm and 660nm, the emitting efficiency is high, the color purity is high, and the strongest emission wavelength is close to the position of the maximum absorption peak of chlorophylls (a, b), so the light conversion agent can meet the physiological requirement of the photosynthesis of plants.
Owner:JIANGSU BOILN PLASTICS CO LTD
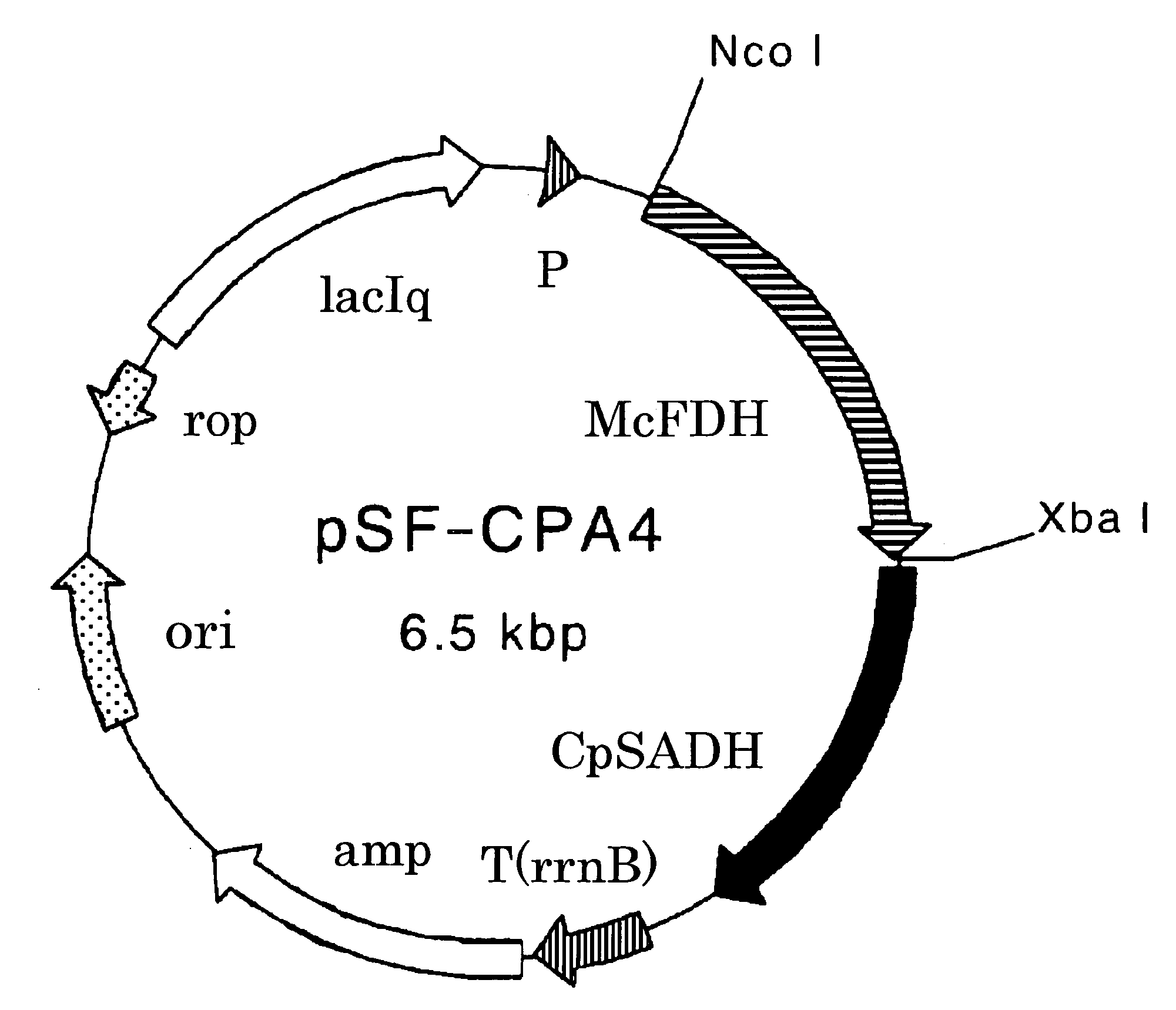
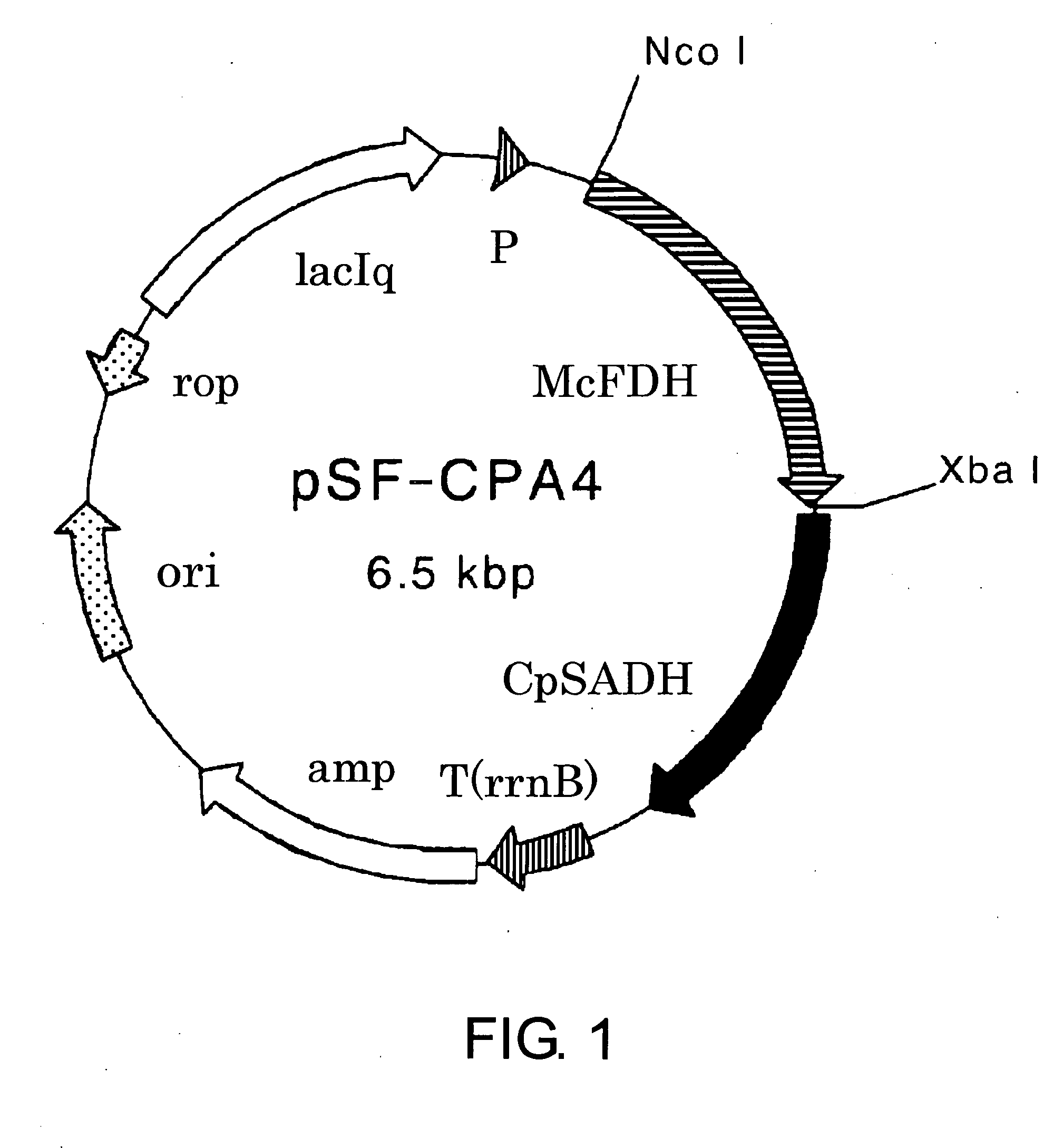
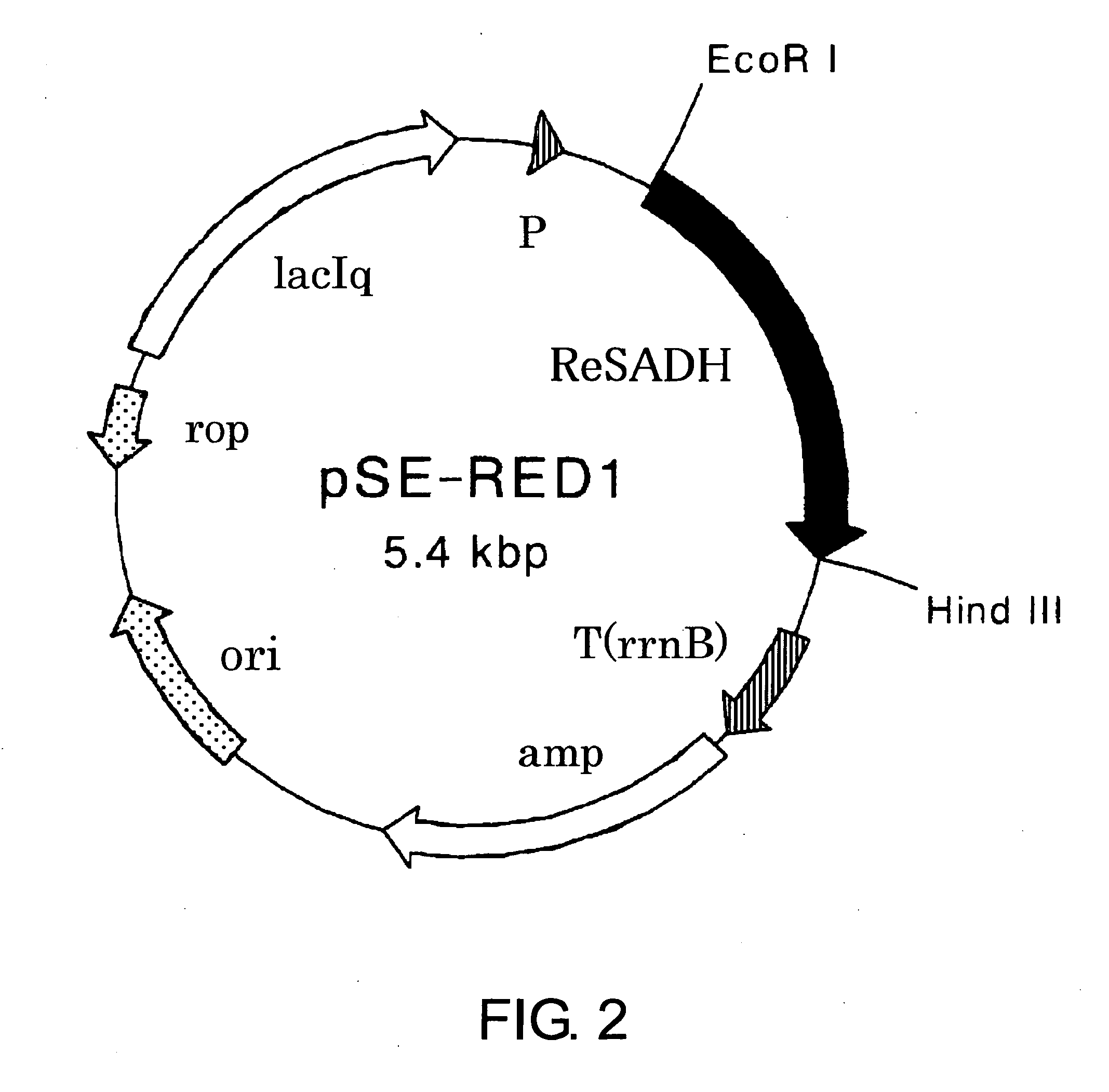
Process for Production of Optically Active Alcohol
The present invention provides methods for producing (S)-1,1,1-trifluoro-2-propanol, which include the step of reacting an enzyme of any one of alcohol dehydrogenase CpSADH, alcohol dehydrogenase ReSADH, carbonyl reductase ScoPAR, (2S,3S)-butanediol dehydrogenase ZraSBDH, carbonyl reductase ScGCY1, tropinone reductase HnTR1, tropinone reductase DsTR1, or alcohol dehydrogenase BstADHT, a microorganism or a transformant strain that functionally expresses the enzyme, or a processed material thereof, with 1,1,1-trifluoroacetone. The present invention also provides methods for producing (R)-1,1,1-trifluoro-2-propanol, which include the step of reacting alcohol dehydrogenase PfODH, a microorganism or a transformant strain that functionally expresses the enzyme, or a processed material thereof, with 1,1,1-trifluoroacetone.
Owner:DAICEL CHEM IND LTD
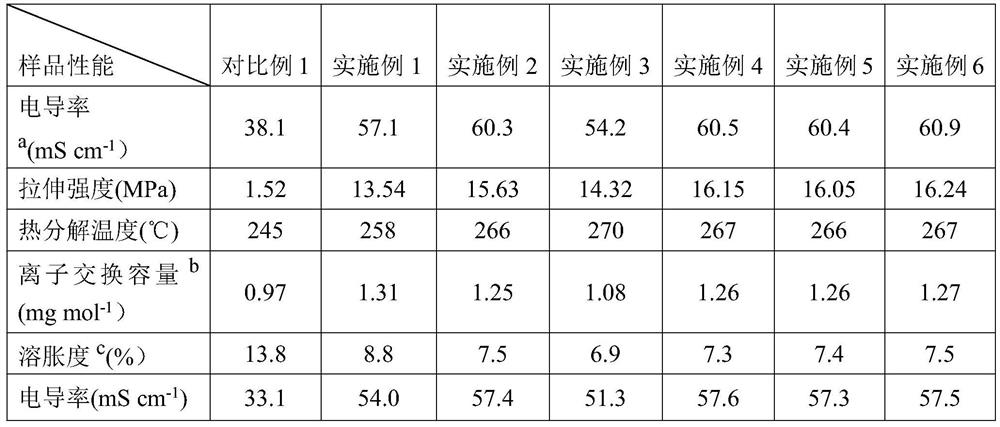
Method for preparing alkaline anion exchange membrane based on branched oxygen-free main chain
A method for preparing an alkaline anion exchange membrane based on a branched oxygen-free main chain belongs to the field of battery membrane materials. The method comprises: firstly, dissolving 1,3,5-triphenylbenzene, N-methylpiperidone, biphenyl and trifluoroacetone in a solvent, adding an catalyst under an ice bath condition, and obtaining a polymer main chain having a branching degree of 1 to5 after a reaction; secondly, dissolving and reacting N-methylpiperidine and 1,6-dibromohexane in ethyl acetate, and then processing an obtained product ionization reagent; finally, performing the ionization of the polymer membrane main chain, casting membrane, and alkali treatment to obtain an anion exchange membrane. The method reduces the risk that hydroxide attacks the membrane by using the oxygen-free main chain, thereby enhancing the alkali resistance of the anion exchange membrane. The branched structure provides a certain free volume to help build ion transmission channels, and the ion reagent can promote the formation of ion clusters as well as the separation of hydrophilic and hydrophobic microphases, thereby significantly improving the conductivity of the membrane.
Owner:DALIAN UNIV OF TECH
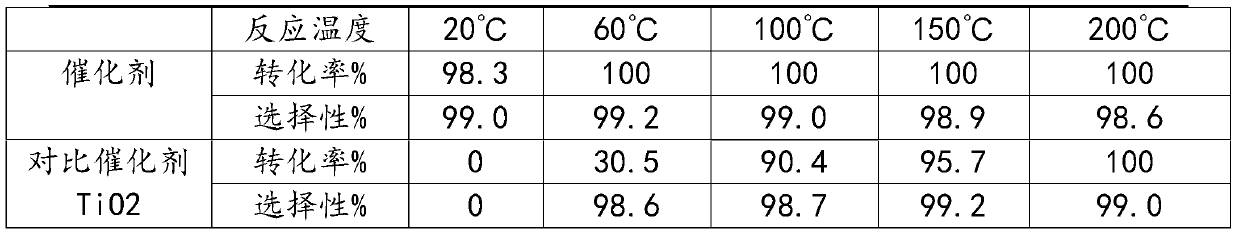
Catalyst for preparing hexafloroacetone by isomerization method and its preparing method and use
ActiveCN1899692ASolve the defect of high requirement for reaction conditionsHigh catalytic activityMetal/metal-oxides/metal-hydroxide catalystsPreparation from heterocyclic compoundsIsomerizationPolymer science
The present invention is catalyst for isomerization process of preparing hexafloroacetone and its preparation and application. The catalyst consists of main catalyst component Cr2O3 and / or AlF3, the first co-catalyst component TiO2 and the second co-catalyst component graphite or metal powder. The catalyst is prepared through mechanical mixing, drying and programmed heating to activate. By means of the catalyst, the isomerization process of preparing hexafloroacetone may be completed under normal temperature and normal pressure, can complete rearrangement instantaneously and has no need of inert gas for diluting material. The catalyst has facile material, simple preparation process, high catalytic activity, high operation stability and capacity of preparing high purity hexafloroacetone.
Owner:SINOCHEM LANTIAN +1
Novel cage type low polysilsesquioxane and rare earth light-emitting material thereof
InactiveCN103012464AGood luminous performanceEasy to produce coordinationSilicon organic compoundsLuminescent compositionsRare-earth elementChemistry
The invention discloses a cage type low polysilsesquioxane and a rare earth ion light-emitting material. 1,3,5,7,9,11,14-heptaisobutyltricyclo[7.3.3.15,11]heptasiloxane-endo-3,7,14-triol is used as a matrix, and an alpha-thenoyltrifluoroacetone silanization derivative, a dipyridyl silanization derivative and a terpyridyl silanization derivative are used as angle complementing bodies, and the complementing bodies react with the matrix in an angle complementing manner to form the complete novel cage type low polysilsesquioxane; and the novel cage type low polysilsesquioxane is combined with a rare earth element to form a cage type low polysilsesquioxane (POSS) / rare earth ion light-emitting material. The rare earth compound / low polysilsesquioxane material is rich in light-emitting colors, is high in color purity, long in fluorescence lifetime (0.5-1.5ms), high in quantum efficiency (20), and strong in heat stability (350 DEG C) and light stability, is a valuable optical material, and can be applied to the fields of display and development, new light sources, X ray intensifying screens and the like.
Owner:HEBEI UNIV OF TECH
Preparation method of cross-linked polyfluorene piperidine anion exchange membrane
The invention discloses a preparation method of a cross-linked polyfluorene piperidine anion exchange membrane. The method comprises the following steps: dissolving 9,9-dimethyl fluorene, N-methyl piperidone and trifluoroacetone in dichloromethane to obtain a homogeneous solution, adding trifluoroacetic acid and trifluoromethanesulfonic acid as catalysts, performing reacting for 24-36 hours at 0-10 DEG C in a nitrogen atmosphere, performing precipitating with a K2CO3 aqueous solution, and performing washing with deionized water to obtain light yellow solid polyfluorene piperidine polymer powder; dissolving the obtained polyfluorene piperidine and iodomethane in chloroform, performing heating and refluxing for 24-36 hours under a dark condition and a nitrogen atmosphere, then pouring a reaction solution into diethyl ether to precipitate, and performing washing with deionized water to obtain a quaternized polyfluorene piperidine polymer; and dissolving the obtained solution in dimethyl sulfoxide, adding a brominated amino siloxane cross-linking agent solution, performing reacting for 12-24 hours at 50-60 DEG C, adding iodomethane, performing reacting for 24-48 hours at 30-40 DEG C in a dark nitrogen atmosphere, adding ethanol, performing standing for 24-36 hours at room temperature, performing standing for 12-24 hours at 80 DEG C, standing for 3-6 hours at 100 DEG C, and performing standing for 1-3 hours at 120 DEG C to obtain the cross-linking type polyfluorene piperidine anion exchange membrane.
Owner:WUHAN UNIV OF TECH
Preparing method of 3-fluoroacetone acid
ActiveCN107056606AThe reaction process conditions are simpleSimple and fast operationOrganic compound preparationCarboxylic acid esters preparationDistillationGas phase
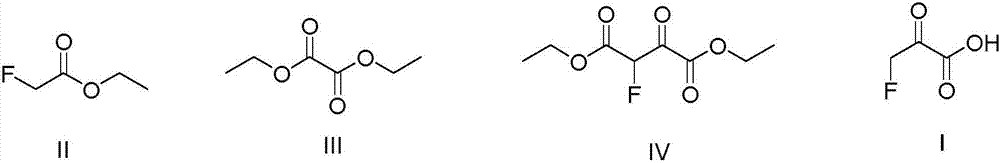
The invention relates to a preparing method of 3-fluoroacetone acid. Diethyl oxalate and fluoro ethyl acetate are adopted as raw materials, NaH is adopted as a strong base catalyst to conduct an ester condensation reaction to obtain 2-fluo-3-oxo diethyl succinate, wherein the yield rate is 84.6%, and the gas phase chromatographic detection purity is 99.9%. 2-fluo-3-oxo diethyl succinate is put into an acid solution, and then through hydrolysis and decarboxylation reactions, and then through distillation and water removal, a coarse product is obtained, and finally through a sublimation method, 3-fluoroacetone acid is obtained, the yield rate is 85.4%, the liquid phase chromatographic detection purity is 99.9%, and the total yield through the two steps is 72%. The reaction technology is simple in condition, reactions are all conventional reactions under the normal pressure, the operation is simple and convenient, the raw materials are easy to obtain, and the product cost is low.
Owner:ZHEJIANG UNIV OF TECH
Method for preparing 1,1,1-trifluoroacetone
InactiveCN102476984ANo explosion hazardPromote safe productionOrganic compound preparationCarbonyl compound preparationOrganic acidEnvironmental engineering
The invention discloses a method for preparing 1,1,1-trifluoroacetone, which is used for preparing the 1,1,1-trifluoroacetone by reacting 4,4,4-trifluoroethyl acetoacetate and organic acid in an anhydrous state. Compared with the prior art, the method overcomes the risk in exploding trifluoroacetone hydrate easily generated in a water reaction environment in air, and has a safe and reliable production process; meanwhile, both the product purity and the yield are higher; furthermore, the organic acid as a raw material can be recycled; the method is low in three-waste pollution, low in production cost and simple to operate; and the prepared 1,1,1-trifluoroacetone can be used for producing a plurality of important fine chemical intermediates.
Owner:SINOCHEM LANTIAN +1
Method for preparing 1,1,1,3,3,3-hexafluoroacetone through gas-phase catalysis
InactiveCN102964231ASimple processLow costOrganic compound preparationCarbonyl compound preparationHydrogen fluoridePtru catalyst
The invention provides a method for preparing 1,1,1,3,3,3-hexafluoroacetone through gas-phase catalysis, relating to the field of organic synthesis. The method comprises the following reaction steps: (1) introducing 1,1,3,3-tetrafluoro-1,3-dichloroacetone gas and hydrogen fluoride gas into a fixed bed type reactor with a supported catalyst, and reacting; and (2) rectifying the gas discharged from the fixed bed type reactor to obtain the 1,1,1,3,3,3-hexafluoroacetone. According to the method for preparing 1,1,1,3,3,3-hexafluoroacetone through gas-phase catalysis, the tetrafluorodichloroacetone is used as the raw material; and the process is simple and environment-friendly, low in cost and high in yield.
Owner:NANJING UNIV OF INFORMATION SCI & TECH
Asymmetric hydrogenation of 1,1,1-trifluoroacetone
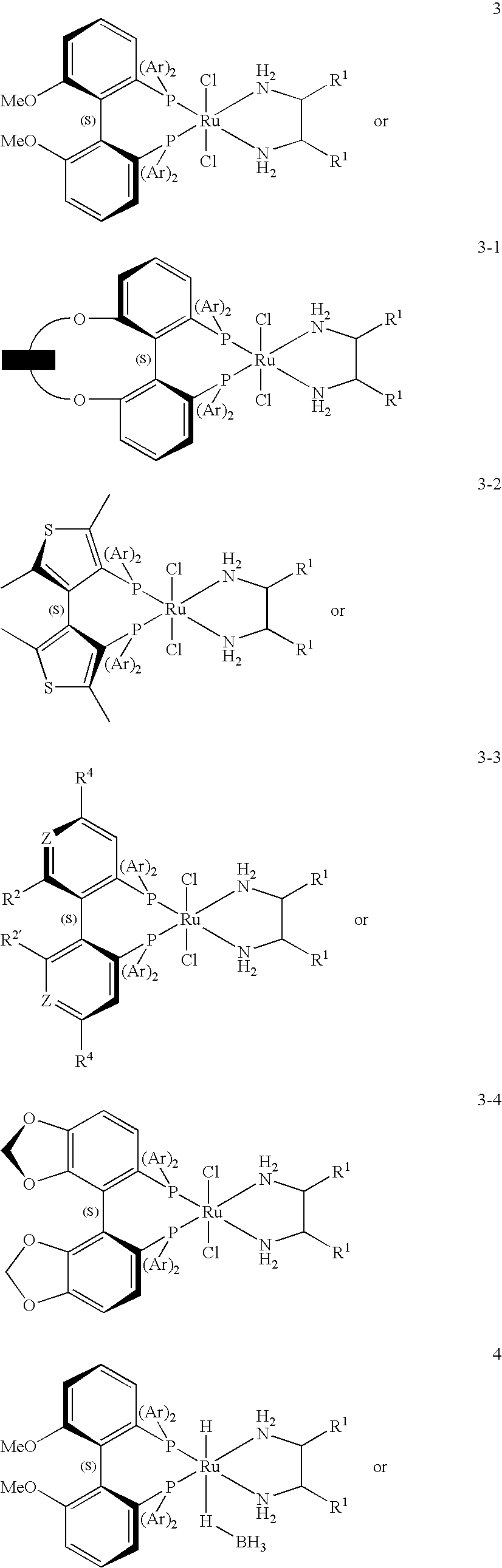
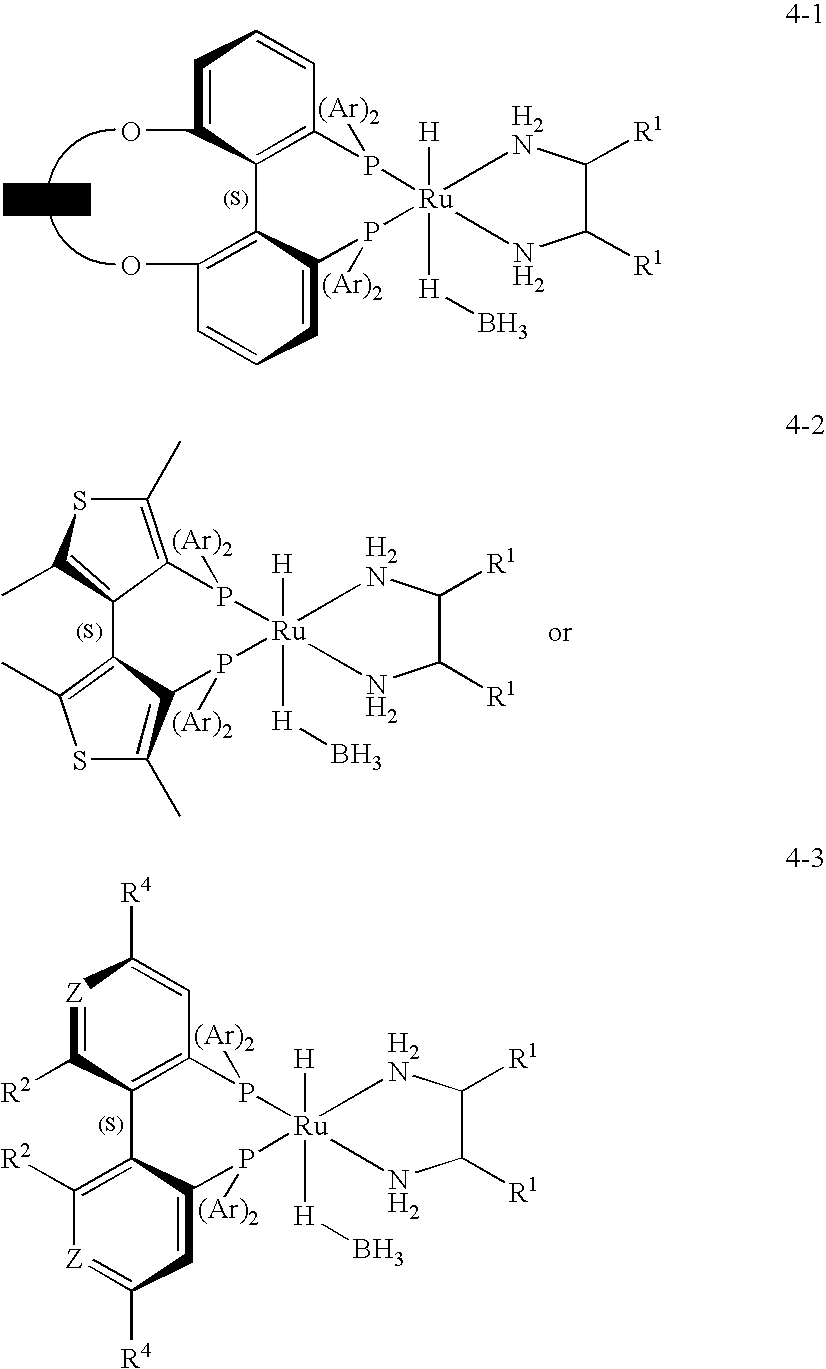
The invention relates to the preparation of enantiomerically pure (S)-1,1,1-trifluoro-2-propanol by asymmetric hydrogenation of 1,1,1-trifluoroacetone which process comprises hydrogenating 1,1,1-trifluoroacetone in the presence of a ruthenium phosphine complex catalyst represented by formulaRu(E)(E′)(L)(A)wherein E, E′ are both chloro or E is hydrogen and E′ is BH4;L is a chiral diphosphine ligand; andA is an optionally chiral diaminewherein hydrogenation occurs in the presence of a weak base, with or without an additive, when E and E′ are both chloro orb) in the absence of a base and an additive when E and E′ are hydrogen and BH4.
Owner:F HOFFMANN LA ROCHE & CO AG
Preparation method of modified time-resolved fluorescent microspheres
PendingCN111218270ATime resolution does not affectResolution does not affectBiological testingLuminescent compositionsPotassium persulfatePolystyrene
The invention discloses a preparation method of modified time-resolved fluorescent microspheres. The preparation method comprises the following steps: (1) adding ultrapure water into a reaction kettle, introducing nitrogen, stirring while heating, adding rectified polystyrene, mixing and heating, then adding potassium persulfate, and fully reacting to obtain white latex spheres; and (2) weighing asolvent dye, 2-thiophene formyl trifluoroacetone, phenanthroline hydrochloride, europium chloride and an electron mediator, dissolving the solvent dye, the 2-thiophene formyl trifluoroacetone, the phenanthroline hydrochloride, the europium chloride and the electron mediator in petroleum ether to generate a mixture, slowly adding the mixture into a white latex microsphere solution prepared in step(1), uniformly stirring to obtain dyeing time-resolved fluorescent microspheres, heating to completely volatilize petroleum ether, and repeatedly centrifugally washing the synthesized microspheres tocompletely wash away redundant dyes in the solution in order to obtain the microspheres with bright colors and without influencing the time-resolved fluorescence of the microspheres. A user does notneed to read numerical values through an instrument when using the microspheres, and the reason of abnormality can be visually found out for an abnormal test strip.
Owner:长沙美牛生物科技有限公司
Cage type oligomeric silsesquioxane prepared by taking BipySi as supplement body and rare earth luminescent material prepared from cage type oligomeric silsesquioxane
InactiveCN104478922AEasy to produce coordinationImprove luminositySilicon organic compoundsLuminescent compositionsRare-earth elementFluorescence
The invention relates to a cage type oligomeric silsesquioxane prepared by taking BipySi as supplement bodies and a rare earth luminescent material prepared from cage type oligomeric silsesquioxane. By taking 1,3,5,7,9,11,14-heptaisobutyl tricyclic[7,3.3.15,11] heptatrisiloxane-intra-3,7,14-triol as a matrix, alpha-thenoyl trifluoroacetone silanized derivative, a dipyridine silanized derivative and a terpyridyl silanized derivative as supplement bodies, the supplement bodies react with the matrix in form of supplements to form integrated novel cage type oligomeric silsesquioxane. The cage type oligomeric silsesquioxane is combined with rare earth elements to form a POSS / rare earth ion luminescent material. The obtained rare earth ion luminescent material / POSS is rich in luminescent color, high in color purity, long in fluorescent lifetime (0.5-1.5ms), high in quantum efficiency (20) and strong in thermal stability (350 DEG C) and light stability, is a valuable optical material and can be applied to the field of display and development, light source, X-ray intensifying screen and the like.
Owner:HEBEI UNIV OF TECH
Preparation method of 4, 4 '-(hexafluoroisopropylidene) diphthalic anhydride
ActiveCN110963985AReduce pollutionOmit the methyl oxidation stepOrganic chemistryPtru catalystEngineering
The invention discloses a preparation method of 4, 4 '-(hexafluoroisopropylidene) diphthalic anhydride. The method comprises the following steps of (a) accurately weighing N-alkyl phthalimide and a catalyst, stirring and raising the temperature to 90-110 DEG C, and dissolving; slowly dropwise adding hexafluoroacetone hydrate, raising the temperature to 100 to 130 DEG C, reacting for 22 to 80 hourswhile stirring, and discharging to obtain 4, 4-(hexafluoroisopropyl) bis (N-alkyl phthalimide); (b) adding alkali to hydrolyze, and acidifying to obtain hexafluorotetracid; and (c) dehydrating to obtain the 4, 4 '-(hexafluoroisopropylidene) diphthalic anhydride. According to the preparation method, the hexafluoroacetone and specific N-alkyl phthalimide directly react under the action of a specific catalyst to prepare hexafluorodianhydride, the step of oxidizing methyl in a traditional method is omitted, the use of metal oxides is avoided, the environmental pollution is small, a normal-pressure reaction is adopted, a high-pressure reaction is not needed, the reaction risk is reduced, the purity of the prepared 4, 4 '-(hexafluoroisopropylidene) diphthalic anhydride is high, and the yield reaches 87% or above.
Owner:珠海派锐尔新材料有限公司
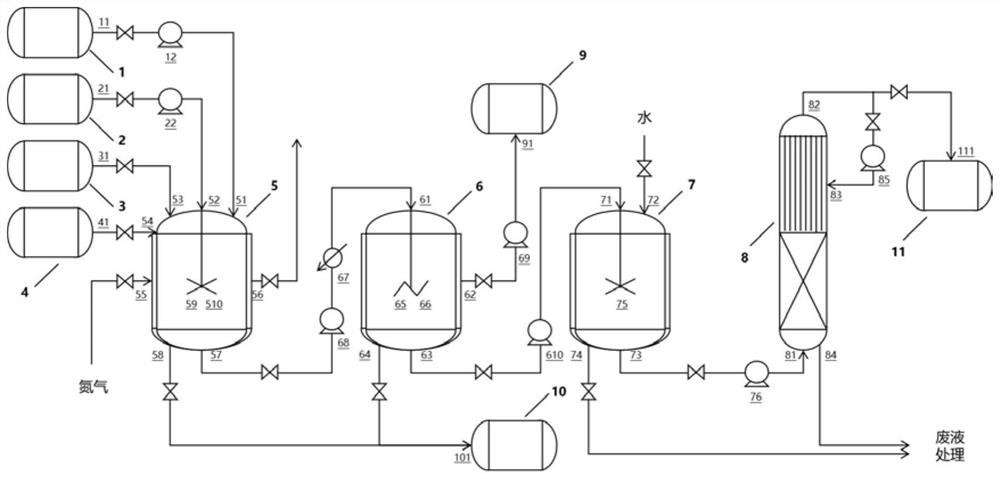
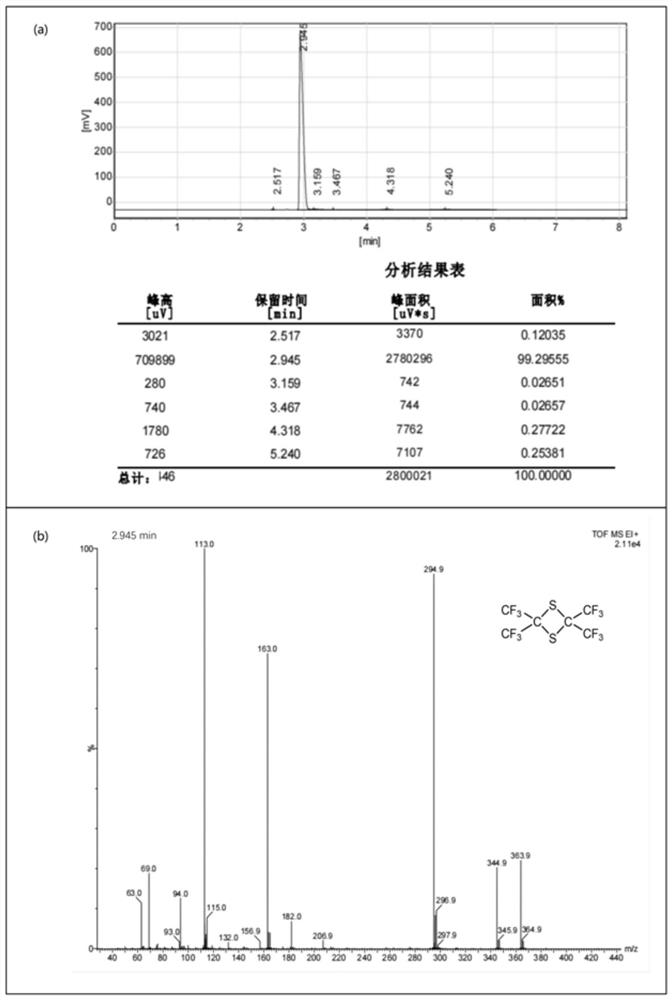
Dipolythiohexafluoroacetone production device and production process
ActiveCN114100551AHigh purityReduce processing costsOrganic chemistrySolution crystallizationTemperature controlHexafluoropropylene
The invention discloses a dimeric thiohexafluoroacetone production device and a production process, which adopt a kettle type reaction device which takes hexafluoropropylene as a raw material and is provided with a corrosion-resistant coating, and are provided with a temperature controller, a heat exchanger and the like, so that the durability and the safety are guaranteed, and the production efficiency is improved. The dimeric thiohexafluoroacetone product with the yield of 95% or above and the purity of 99% or above is obtained through a purification method combining crystallization, water washing and rectification, and the problems that in the prior art, the requirements for high temperature and high pressure are met, energy consumption is large, potential safety hazards exist, waste liquid treatment is difficult, the conversion rate of hexafluoropropylene is not high, and the purity of the product is low can be effectively solved. The solvent and the sulfur solid recovered after separation and purification can be continuously used for the subsequent preparation process, the conversion rate of the hexafluoropropylene raw material is greatly improved, meanwhile, the treatment cost of the waste liquid is reduced, and the method is simple in process route and device, short in reaction time, high in product purity, environmentally friendly and suitable for industrial application.
Owner:ZHEJIANG UNIV OF TECH
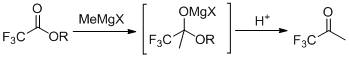
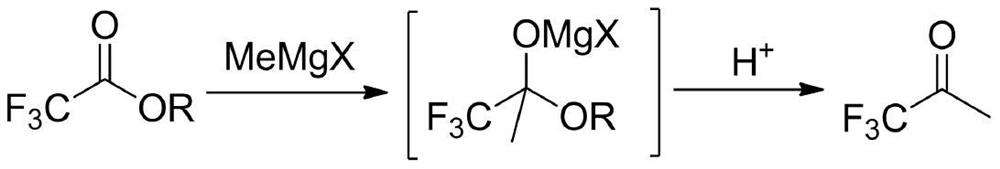
The preparation method of 1,1,1-trifluoroacetone
ActiveCN109942393BRaw materials are easy to getEasy to purify after treatmentOrganic compound preparationCarbonyl compound separation/purificationGrignard reagentBiochemical engineering
Owner:赵博佑
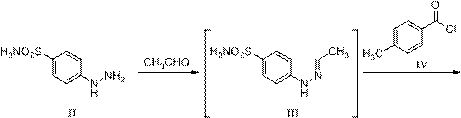
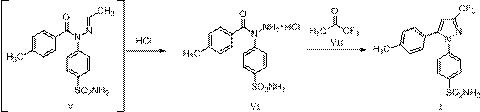
A kind of preparation method of celecoxib
ActiveCN110526868BAvoid generatingMild reaction conditionsOrganic chemistryFluoroacetoneMethyl palmoxirate
The invention relates to a preparation method of celecoxib, which belongs to the technical field of preparation methods of raw materials. The preparation method of celecoxib described in the present invention, at first described in formula II 4-hydrazinobenzenesulfonamide reacts with acetaldehyde, obtains the reaction liquid containing the compound of formula III; Then in the reaction liquid obtained in the first step, add formula The p-toluoyl chloride described in IV can obtain the reaction solution containing formula V; in the reaction solution obtained in the second step, add hydrochloric acid ethanol to obtain compound VI, and then form a plug with 1,1,1-trifluoroacetone ring closure Lecoxib. The change of the ring mode fundamentally avoids the formation of regioisomer impurities. This preparation method uses a one-pot reaction for upprotection, condensation, and deprotection. The reaction conditions are mild, the post-treatment is easy, and the total yield can reach 90%. The above is more suitable for large-scale industrial production.
Owner:迪嘉药业集团股份有限公司
Copolymerized polystyrene fluorescent microsphere and preparation method thereof
ActiveCN114853934AGood fluorescenceGood monodispersityLuminescent compositionsImmune profilingPhenanthroline
The invention discloses a copolymerized polystyrene fluorescent microsphere and a preparation method thereof, a fluorescent substance is a rare earth europium complex, and the rare earth europium complex takes a phenanthroline derivative introduced with double bonds as a second ligand and beta-naphthoyl trifluoroacetone as a first ligand. The rare earth europium complex and styrene are initiated by an initiator in a solvent, the fluorescent microspheres are obtained through free radical copolymerization, and the content of the europium complex in the fluorescent microspheres is regulated and controlled to obtain the optimal fluorescence performance. The copolymerized fluorescent microspheres disclosed by the invention have the advantages of good dispersity, uniform particle size, good luminous effect, high fluorescence intensity, long fluorescence lifetime and the like. The copolymerization type fluorescent microspheres are good in stability, overflow of internal fluorescent substances is avoided, and technical support is provided for application of the fluorescent microspheres in the fields of immunoassay, standard substance biological probes and the like.
Owner:SOUTHEAST UNIV
Method for analyzing and determining content of hexafluoroacetone
ActiveCN111579669AHigh utility valueEasy to operateComponent separationGas liquid chromatographicQuantitative determination
The invention discloses a method for analyzing and determining the content of hexafluoroacetone. Hexafluoroacetone is subjected to gas chromatographic analysis, the content of hexafluoroacetone is directly and quantitatively determined by an area normalization method, and chromatographic conditions during detection are as follows: a chromatographic column is a non-polar column; the temperature ofa chromatographic column is 40-200 DEG C; the temperature of a sample inlet is 60-140 DEG C; the temperature of a detector is 250 to 300 DEG C; the carrier gas is high-purity nitrogen; the flow rate of carrier gas is 0.8 mL / min to 1.5 mL / min; the split ratio is (30-100): 1; the operation time is 20 min, and the pre-column pressure is 13 Psi; and the sample size is 0.1 to 1.0 mL. And the detector is a hydrogen flame detector. The chromatographic analysis method for directly and quantitatively determining the purity of hexafluoroacetone in production has the advantages of reliable result, simpleoperation and low test price, is suitable for routine analysis of enterprises, has high practical value, and can be used for guiding the research and production process detection of hexafluoroacetone.
Owner:SHANDONG DONGYUE WEILAI HYDROGEN ENERGY MATERIAL CO LTD
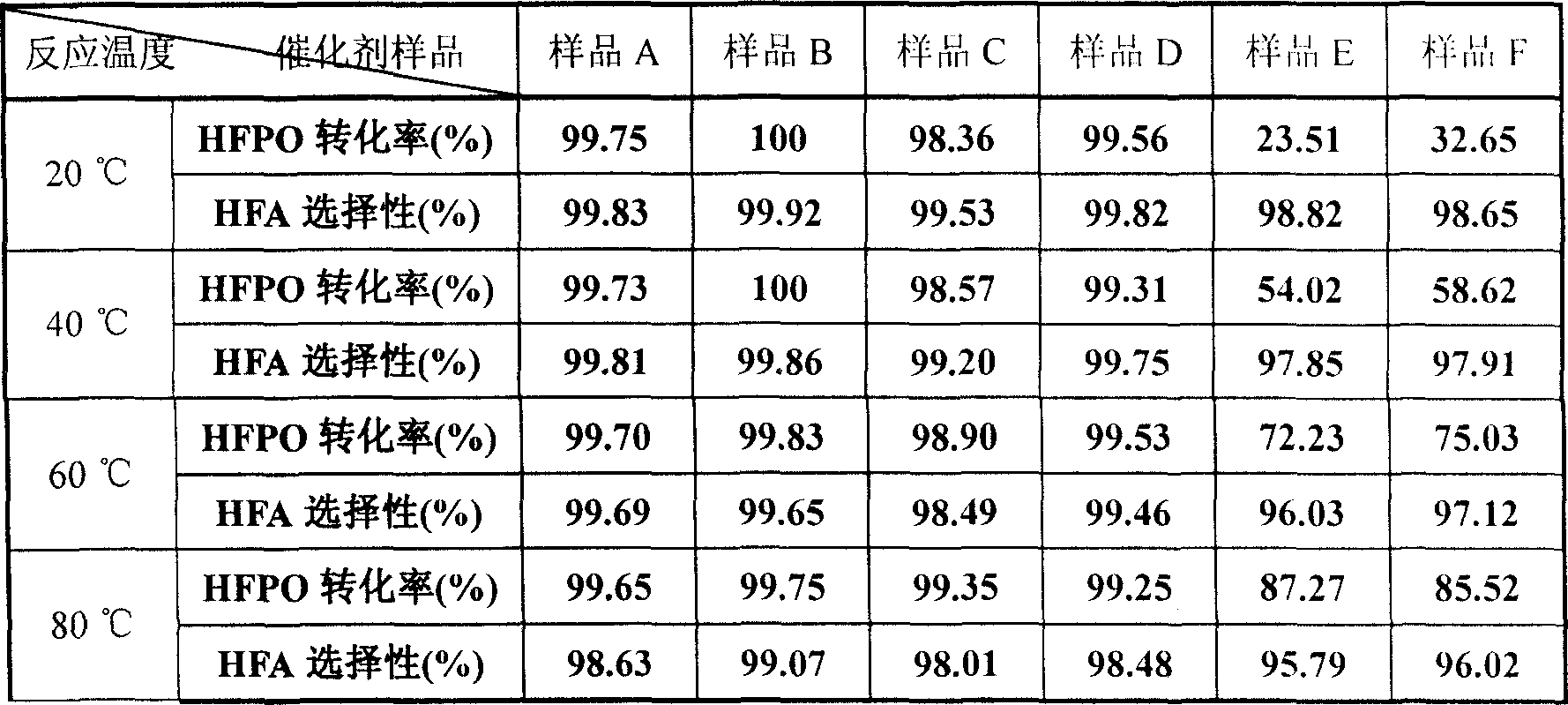
Method for preparing hexafluoroacetone by taking perfluoropropylene oxide as raw material
ActiveCN111004099ALow costSimple preparation processPreparation from heterocyclic compoundsPtru catalystIsomerization
The invention discloses a method for preparing hexafluoroacetone by taking perfluoropropylene oxide as a raw material. The method comprises the following steps: adding the perfluoropropylene oxide, acatalyst and water into a reaction kettle according to a weight ratio of 1: (0.1-0.5): (1-5), carrying out isomerization reaction at 10-80 DEG C for 1-5 hours, and distilling and purifying to obtain the hexafluoroacetone, wherein by condensing substituted phenol and Merrifield resin, and mixing and compounding with a carrier, the catalyst is obtained. According to the method, the Merrifield resinloaded substituted phenol is used as the catalyst for the first time, and is applied to the method for preparing the hexafluoroacetone by taking the perfluoropropylene oxide as the raw material, so that a new preparation thought is provided, and particularly for fluorine chemical enterprises, self-produced intermediate products can be fully utilized. The raw materials of the catalyst are easy to obtain, the cost is low, and the economic benefit is good; the catalyst is simple in preparation process and mild in preparation condition. The yield of the hexafluoroacetone reaches 95% or above, theoperation is safe, and the catalyst can be continuously used.
Owner:浙江利化新材料科技有限公司
Preparation method of tralopyril
PendingCN113880745AThe synthetic route is simpleRaw materials are easy to obtainOrganic chemistryAlkaneChlorobenzene
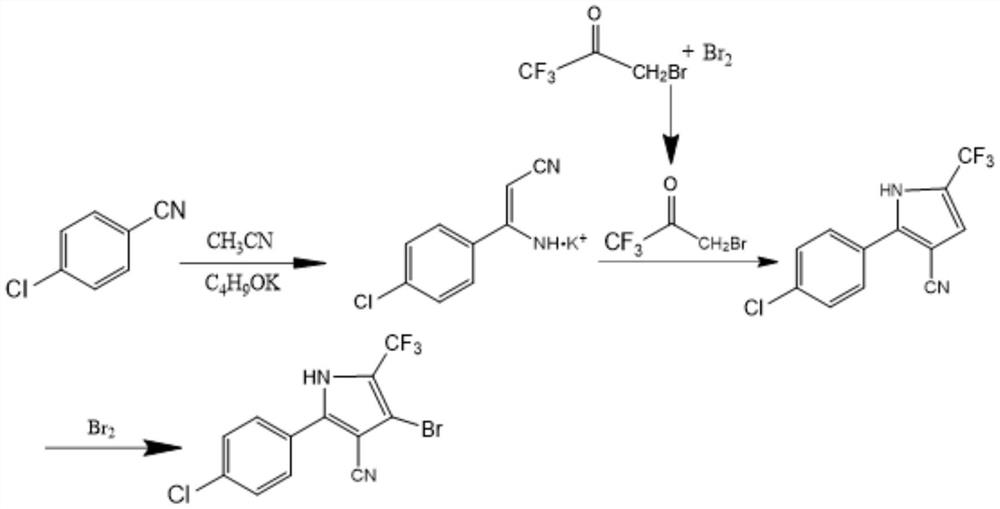
The invention discloses a preparation method of tralopyril, and particularly relates to the technical field of agriculture. The preparation method provided by the invention comprises the steps of 1, dissolving p-chlorobenzonitrile in acetonitrile, and carrying out a salt forming reaction under the catalysis of an organic base to obtain an alkali metal salt (I) of p-chlorophenyl amino acrylonitrile; 2, dissolving 1, 1, 1-trichloroacetone in alkyl chloride, carrying out a one-step bromination reaction with bromine, and carrying out extraction and distillation to obtain 1-bromo-3, 3, 3-trifluoroacetone (II); 3, dissolving the intermediate I in alkane, carrying out a ring closing reaction with the intermediate II through acid catalysis to obtain a 2-(4-chlorphenyl)-5-(trifluoromethyl)-1H-pyrrole-3-nitrile crude product, and carrying out distillation and recrystallization to obtain a pure product; and 4, performing two-step bromination on the intermediate III through bromine to obtain tralopyril. According to the intermediate obtained by the preparation method disclosed by the invention, the final product tralopyril is low in impurity content and high in product purity, the raw materials are simple and easy to obtain, the preparation process is simple, the reaction conditions are mild, and the preparation method is economical and environment-friendly and meets the requirements of industrial mass production.
Owner:山东亿嘉农化有限公司
Fluorinated silicon-aluminum molecular sieve as well as preparation method and application thereof
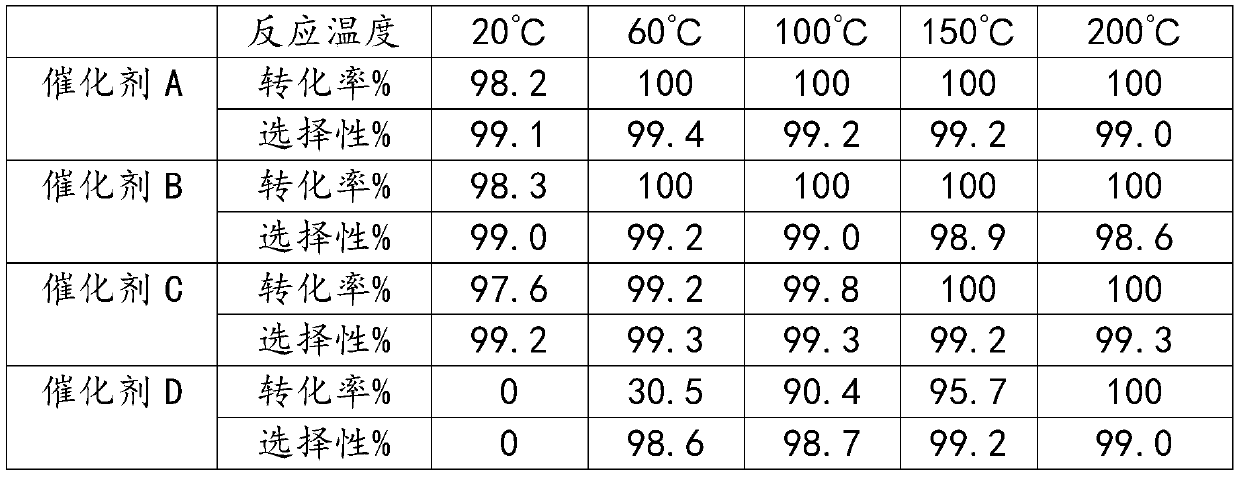
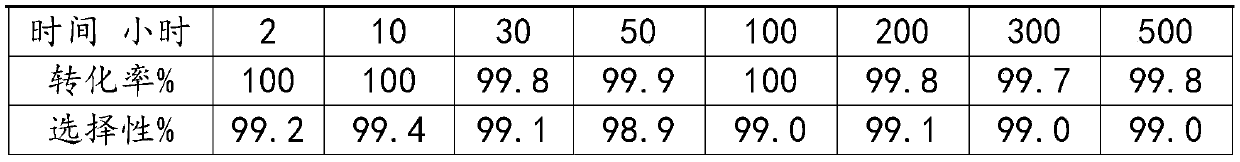
ActiveCN111017953AHigh catalytic efficiencyHigh selectivityMolecular sieve catalystsCatalyst activation/preparationMolecular sieveFluorinated gases
The invention belongs to the field of catalyst preparation, and particularly relates to a fluorinated silicon-aluminum molecular sieve as well as a preparation method and an application thereof. The preparation method of the fluorinated silicon-aluminum molecular sieve comprises the following steps: drying a molecular sieve in an oven, putting the molecular sieve into a tubular furnace, introducing N2, heating to 200-500 DEG C, introducing CHClF2 gas for fluorination, introducing N2 after fluorination, and cooling to room temperature; and tabletting and molding to obtain 20-40 meshes. The fluorinated gas is introduced into a tubular furnace through CHClF2 (R22) and reacts with the molecular sieve to form the fluorinated silicon-aluminum molecular sieve, operation is convenient, and the obtained silicon-aluminum molecular sieve serving as a catalyst for preparing hexafluoroacetone is high in catalytic efficiency, high in selectivity and high in stability.
Owner:TIANJIN CHANGLU CHEM NEW MATERIAL CO LTD
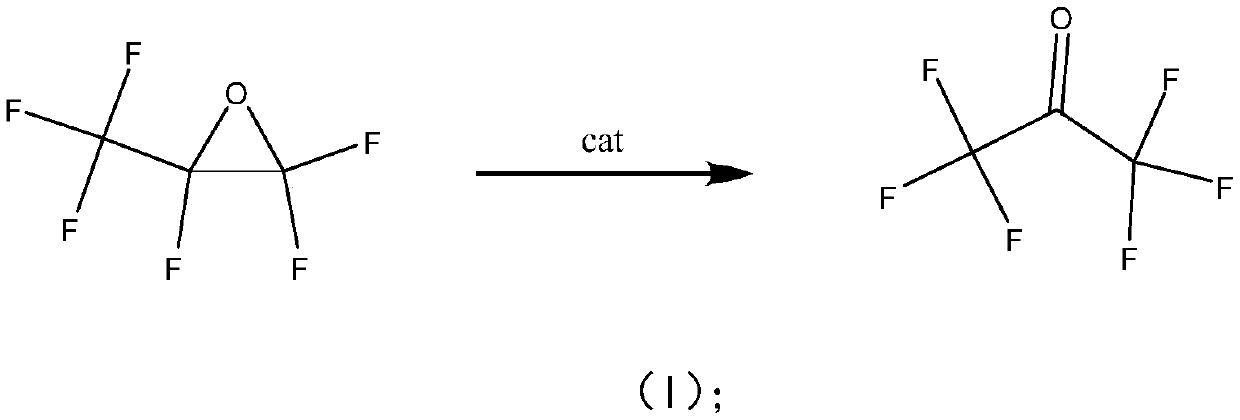
Method for preparing hexafluoroacetone by isomerizing hexafluoropropylene oxide
ActiveCN111116342AEasy to makeRaw materials are easy to getPreparation from heterocyclic compoundsPtru catalystReaction temperature
The invention belongs to the field of fluoride preparation, and particularly relates to a method for preparing hexafluoroacetone through isomerizing hexafluoropropylene oxide. The method specificallycomprises the following steps: filling a reactor with a catalyst, introducing the reaction raw material gas hexafluoropropylene oxide, carrying out a reaction at a reaction pressure of normal pressure, a reaction temperature of 0-200 DEG C and a reaction space velocity of 100-600 h <-1 >, and introducing the product into ionized water after the reaction is finished so as to obtain hexafluoroacetone hydrate. According to the method disclosed by the invention, high-purity hexafluoroacetone can be obtained by isomerizing the hexafluoropropylene oxide, almost no by-product is formed, the selectivity is high, the reaction can be carried out at low temperature and normal pressure, and the requirement on reaction conditions is low. Meanwhile, the catalyst used in the invention is simple to prepare, easily available in raw materials, easy to form and suitable for large-scale production.
Owner:TIANJIN CHANGLU CHEM NEW MATERIAL CO LTD
Cable outer layer insulation material and preparation method thereof
InactiveCN104927317AHigh mechanical strengthImprove insulation performancePlastic/resin/waxes insulatorsPolymer sciencePolyvinyl alcohol
The invention discloses a cable outer layer insulation material and a preparation method thereof. The cable outer layer insulation material is prepared by using the following components in parts by weight: 78-95 parts of polyethylene glycol terephthalate, 26-33 parts of polyvinyl butyral, 15-25 parts of bisphenol hexafluoroacetone diglycidyl ether, 3-8 parts of tourmaline powder, 2-6 parts of hexamethylcyclotrisiloxane, 3-5 parts of bis-(phenyl dimethyl siloxane) methylsiliconate, 1-2 parts of polysebacic urea, 0.02-0.5 parts of benzoyl peroxide butyl acetate and 0.01-0.5 parts of copper chloride. The invention further provides a preparation method for the cable outer layer insulation material.
Owner:SUZHOU KEMAO ELECTRONICS MATERIALS TECH
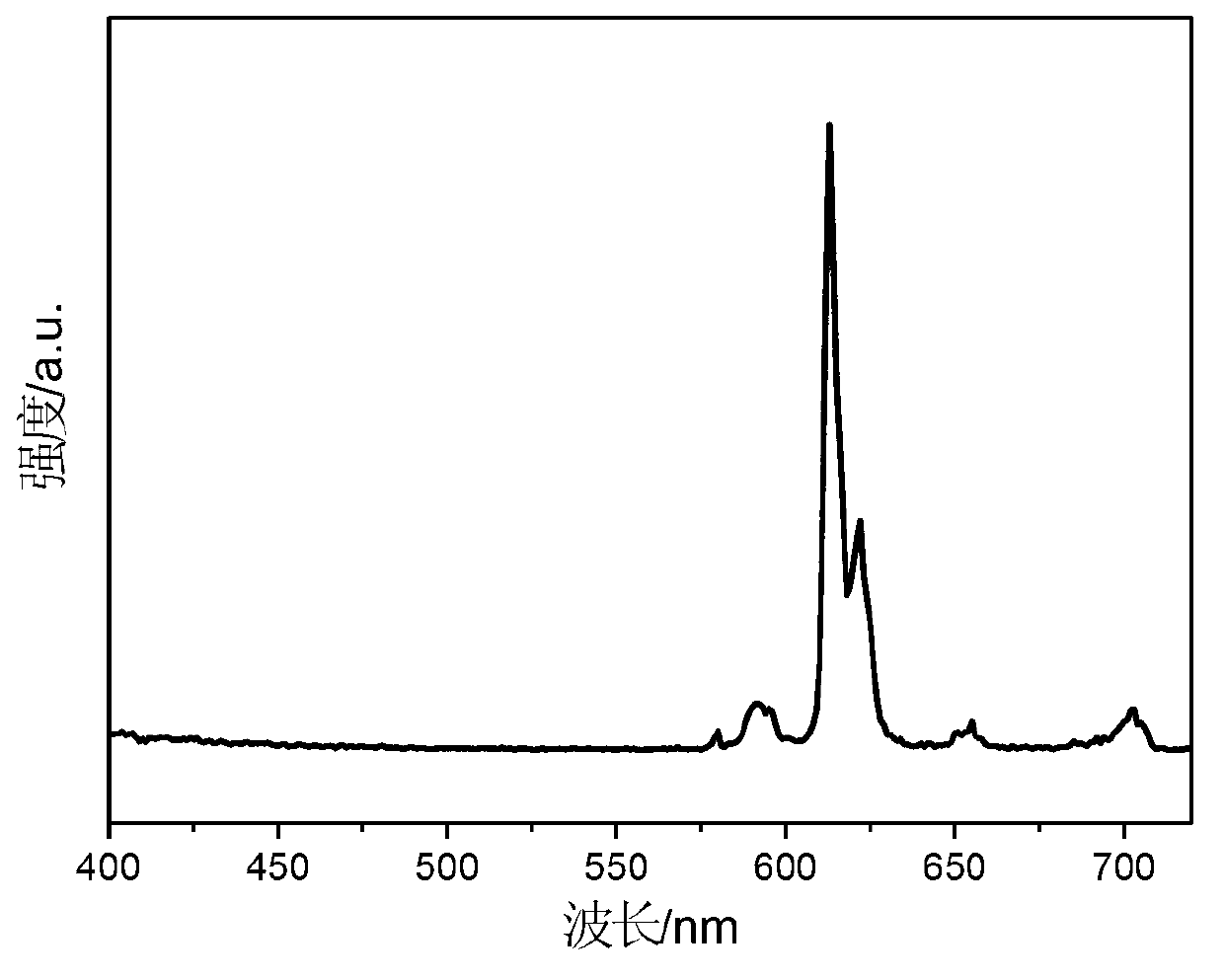
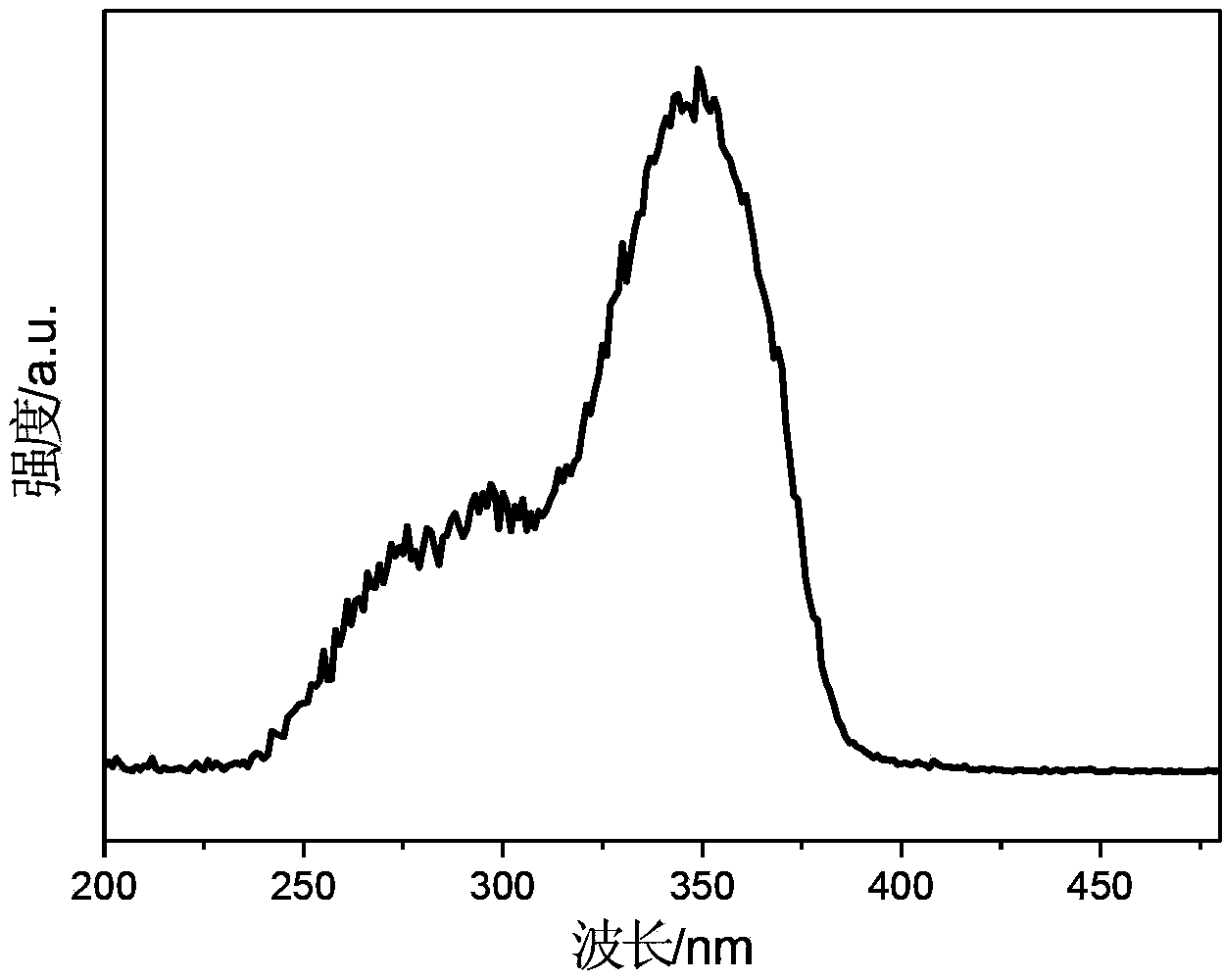
High-sensitivity rare earth doped time-resolved fluorescent nanoparticle, and preparation method and application thereof
ActiveCN113249127AFluorescence enhancementEliminate quenching factorsMaterial nanotechnologyNanoopticsThenoyltrifluoroacetonePhenanthroline
Owner:HUAZHONG UNIV OF SCI & TECH +1
A kind of biodegradable light conversion film and preparation method thereof
ActiveCN109251488BEffective absorptionMeet growth needsClimate change adaptationPlant protective coveringsPolymer sciencePolybutylene
The invention provides a biodegradable light conversion film, which is made of the following components in parts by weight: polybutylene adipate-terephthalate: 60-64.7 parts; polylactic acid: 35 parts; lubricating agent: 0.1-2 parts; opening agent: 0.1-2 parts; light-changing agent: 0.1-1 part; the light-changing agent is an organic compound formed by rare earth europium, α-thienoyl trifluoroacetone and triphenylphosphine Complexes. The invention uses polybutylene adipate-terephthalate and polylactic acid as the polymer matrix, and synthesizes a light-changing agent with rare earth europium and organic small molecule ligands, which can effectively absorb ultraviolet light and emit red light in line with the requirements of plants. Growth requirements, and at the same time have biodegradable properties, reduce the accumulation of mulch film in the soil arable layer. The degradable light-converting film of the present invention has a wide range of applications, and can replace traditional PE mulch films for crop cultivation, and has broad market prospects. The invention also provides a preparation method of the biodegradable light conversion film.
Owner:CHANGCHUN INST OF APPLIED CHEMISTRY - CHINESE ACAD OF SCI
A kind of method for preparing hexafluoroacetone with perfluoropropylene oxide as raw material
ActiveCN111004099BLow costSimple preparation processPreparation from heterocyclic compoundsIsomerizationPtru catalyst
The invention discloses a method for preparing hexafluoroacetone by using perfluoropropylene oxide as raw material. The isomerization reaction is carried out at 10-80 DEG C for 1-5 hours, and then hexafluoroacetone is obtained by distillation and purification. The catalyst is obtained by condensing substituted phenol and Merrifield resin, and then mixing and compounding with the carrier. The invention uses the substituted phenol supported by Merrifield resin as a catalyst for the first time, and is applied to the method for preparing hexafluoroacetone by using perfluoropropylene oxide as a raw material, so as to provide a new preparation idea, especially for fluorine chemical enterprises, which can fully Utilize homegrown intermediates. In addition, the raw materials used in the catalyst are readily available, the cost is low, and the economic benefit is good; the preparation process of the catalyst is simple, and the preparation conditions are mild. The yield of hexafluoroacetone is over 95%, the operation is safe, and the catalyst can be used continuously.
Owner:浙江利化新材料科技有限公司
A green and environmentally friendly method for preparing hexafluoroisobutene by visible light catalysis
The invention discloses a green and environment-friendly method for preparing hexafluoroisobutene by visible light catalysis. The steps include: a) adding photocatalyst into a transparent container, removing oxygen in the transparent container and placing it in a low-temperature dry ice bath; Fluoroacetone and vinyl ketone are stirred evenly; b) under stirring condition, irradiate the reactant in step a) with visible light and c) obtain hexafluoroisobutene product through rectification, separation and purification. The starting material hexafluoroacetone of the present invention is a commercial product, easy to obtain, and low in cost; it is prepared by photocatalysis, and is environmentally friendly; it is prepared by a one-step method, and the process is simple; the raw materials and reactants used are easy to separate, and the purification is simple.
Owner:浙江诺诚技术发展有限公司
Preparation device of hexafluoroacetone hydrate
PendingCN111018683AIncrease productivityImprove conversion rateMolecular sieve catalystsPreparation from heterocyclic compoundsPtru catalystPhysical chemistry
The invention belongs to the field of fluoride preparation devices, and particularly relates to a preparation device of a hexafluoroacetone hydrate, which comprises an HFPO storage tank, a reaction tank and a deionized water tank which are connected in sequence. The reaction tank is filled with a catalyst; a gas outlet in the upper end of the HFPO storage tank is communicated with a gas inlet in the lower end of the reaction tank; a gas outlet formed in the upper end of the reaction tank is communicated with the deionized water tank through a pipeline; an inverted funnel is arranged at the front end of the pipeline; and a liquid outlet is formed in the lower end of the deionized water tank. According to the preparation device of the hexafluoroacetone hydrate, the prepared hexafluoroacetonecan directly generate the hexafluoroacetone hydrate, intermittent collection can be conducted on the hexafluoroacetone hydrate through the design of a partition plate, and the production efficiency is improved.
Owner:TIANJIN CHANGLU CHEM NEW MATERIAL CO LTD
Novel cage type low polysilsesquioxane and rare earth light-emitting material thereof
InactiveCN103012464BEasy to produce coordinationImprove luminositySilicon organic compoundsLuminescent compositionsRare-earth elementFluorescence
The invention discloses a cage type low polysilsesquioxane and a rare earth ion light-emitting material. 1,3,5,7,9,11,14-heptaisobutyltricyclo[7.3.3.15,11]heptasiloxane-endo-3,7,14-triol is used as a matrix, and an alpha-thenoyltrifluoroacetone silanization derivative, a dipyridyl silanization derivative and a terpyridyl silanization derivative are used as angle complementing bodies, and the complementing bodies react with the matrix in an angle complementing manner to form the complete novel cage type low polysilsesquioxane; and the novel cage type low polysilsesquioxane is combined with a rare earth element to form a cage type low polysilsesquioxane (POSS) / rare earth ion light-emitting material. The rare earth compound / low polysilsesquioxane material is rich in light-emitting colors, is high in color purity, long in fluorescence lifetime (0.5-1.5ms), high in quantum efficiency (20), and strong in heat stability (350 DEG C) and light stability, is a valuable optical material, and can be applied to the fields of display and development, new light sources, X ray intensifying screens and the like.
Owner:HEBEI UNIV OF TECH
Cage oligomeric silsesquioxane and its rare earth luminescent material prepared with tpysi as supplementary angle
InactiveCN104530115BEasy to produce coordinationImprove luminositySilicon organic compoundsLuminescent compositionsPolymer scienceFluorescence
The invention relates to a caged oligomeric silsesquioxane prepared with TpySi as an angle supplementing body, and a rare earth luminescent material thereof. A matrix 1,3,5,7,9,11,14-heptylisobutyltricyclo[7.3.3.15.11]heptasiloxane-endo-3,7,14-triol reacts with an alpha-thenoyltrifluoroacetone silanization derivative, a dipyridine silanization derivative and a tripyridine silanization derivative in an angle supplementing manner to form complete novel caged oligomeric silsesquioxane. The novel caged oligomeric silsesquioxane combines with a rare earth element to form a caged oligomeric silsesquioxane (POSS) / rare earth ion luminescent material. The rare earth compound / oligomeric silsesquioxane material has abundant luminescent colors, high color purity, long fluorescence life (of 0.5-1.5ms), high quantum efficiency (of 20), and strong heat stability (at 350DEG C) and light stability, is an optical material with high values, and can be applied in the fields of display, imaging, new light sources and X-ray intensifying screens.
Owner:HEBEI UNIV OF TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com